question_answer 1) Two spherical black bodies of radii\[{{r}_{1}}\]and \[{{r}_{2}}\]and with surface temperature\[{{T}_{1}}\]and\[{{T}_{2}}\] respectively. Radiate the same power. Then the ratio of \[{{r}_{1}}\]and \[{{r}_{2}}\]will be:
A)
\[{{\left( \frac{{{T}_{2}}}{{{T}_{1}}} \right)}^{2}}\]
done
clear
B)
\[{{\left( \frac{{{T}_{2}}}{{{T}_{1}}} \right)}^{4}}\]
done
clear
C)
\[{{\left( \frac{{{T}_{1}}}{{{T}_{2}}} \right)}^{2}}\]
done
clear
D)
\[{{\left( \frac{{{T}_{1}}}{{{T}_{2}}} \right)}^{4}}\]
done
clear
View Answer play_arrow
question_answer 2) A source having frequency of 240 Hz is moving towards on observer with a speed of 20 m/s. when the observer is moving towards the source with a velocity of 20 m/s. Then apparent frequency heard by the observer, if velocity of sound is 330 m/s, will be:
A)
268 Hz
done
clear
B)
271 Hz
done
clear
C)
368 Hz
done
clear
D)
250 Hz
done
clear
View Answer play_arrow
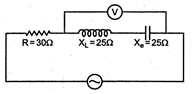
question_answer 3)
In the circuit shown in figure neglecting source resistance the voltmeter and ammeter reading will respectively, will be:
A)
\[~0\text{ }V,3\text{ }A\]
done
clear
B)
\[150\,V,3A\]
done
clear
C)
\[150\,V,6A\]
done
clear
D)
\[0\,V,8A\]
done
clear
View Answer play_arrow
question_answer 4)
A)
\[{{K}_{3}}=\frac{1}{2}({{K}_{1}}+{{K}_{2}})\]
done
clear
B)
\[{{K}_{3}}={{K}_{1}}+{{K}_{2}}\]
done
clear
C)
\[{{K}_{3}}=\frac{{{K}_{1}}{{K}_{2}}}{{{K}_{1}}+{{K}_{2}}}\]
done
clear
D)
\[{{K}_{3}}=2({{K}_{1}}+{{K}_{2}})\]
done
clear
View Answer play_arrow
question_answer 5)
PQRS is a square loop made of uniform conducting wire the current enters the loop at P and leaves at S. Then the magnetic field will be:
A)
maximum at the centre of the loop
done
clear
B)
zero at the centre of loop
done
clear
C)
zero at all points inside the loop
done
clear
D)
zero at all points outside of the loop
done
clear
View Answer play_arrow
question_answer 6) If the velocity of sound in air is 336 m/s. The maximum length of a closed pipe that would produce a just audible sound will be:
A)
\[3.2\,cm\]
done
clear
B)
\[4.2\,m\]
done
clear
C)
\[4.2\,cm\]
done
clear
D)
\[3.2\,m\]
done
clear
View Answer play_arrow
question_answer 7)
A)
full image will be formed but without central portion
done
clear
B)
two image will be formed one due to each exposed half
done
clear
C)
full image will be formed but it is less bright
done
clear
D)
no image will be formed
done
clear
View Answer play_arrow
question_answer 8) A ray of light is incident normally on one of the falls of a prism of angle \[{{30}^{o}}\] and refractive index \[\sqrt{2}.\] The angle of deviation will be:
A)
\[{{26}^{o}}\]
done
clear
B)
\[{{0}^{o}}\]
done
clear
C)
\[{{23}^{o}}\]
done
clear
D)
\[{{13}^{o}}\]
done
clear
View Answer play_arrow
question_answer 9) Translational kinetic energy of a gas molecule, for one mole of the gas, is equal to:
A)
\[2KT\]
done
clear
B)
\[\frac{1}{2}RT\]
done
clear
C)
\[\frac{3}{2}RT\]
done
clear
D)
\[\frac{2}{3}KT\]
done
clear
View Answer play_arrow
question_answer 10) If the radius of earth is reduced by 2% keeping its mass constant. Then the weight of the body on its surface will:
A)
increases
done
clear
B)
decreases
done
clear
C)
remain same
done
clear
D)
either or
done
clear
View Answer play_arrow
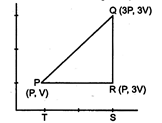
question_answer 11)
An ideal gas is taken around PQRP as shown in the figure of PV diagram. The work done during a cycle is:
A)
zero
done
clear
B)
\[\frac{1}{2}PV\]
done
clear
C)
PV
done
clear
D)
\[2PV\]
done
clear
View Answer play_arrow
question_answer 12) In an as tronomial telescope, the focal length of objective lens and eye piece are 150 cm and 6 cm respectively. In normal adjustment the magnifying power is:
A)
20
done
clear
B)
30
done
clear
C)
60
done
clear
D)
15
done
clear
View Answer play_arrow
question_answer 13) In an experiment with Kund's tube, the distance between consecutive nodes is 5 cm, when air is inside and it is 13-4 cm, when hydrogen is inside then the velocity of sound in hydrogen will be (velocity of sound in air =330 m/s):
A)
\[~884.4\text{ }m/s\]
done
clear
B)
\[984.4\text{ }m/s\]
done
clear
C)
\[1084.4\text{ }m/s\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 14) A second harmonic has to be generated in a string of length I stretched between two rigid supports. The point where the string has to be plucked and touched are:
A)
plucked at\[\frac{l}{4}\]and touch at\[\frac{l}{2}\]
done
clear
B)
plucked at\[\frac{l}{4}\]and touch at \[\frac{3l}{4}\]
done
clear
C)
plucked at\[\frac{l}{2}\] and touched at \[\frac{l}{4}\]
done
clear
D)
plucked at\[\frac{l}{2}\]and touched at\[\frac{3l}{4}\]
done
clear
View Answer play_arrow
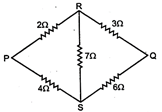
question_answer 15)
Five resistance are connected as shown in figure. The equivalent resistance between P and Q will be:
A)
\[\frac{10}{3}\Omega \]
done
clear
B)
\[\frac{20}{3}\Omega \]
done
clear
C)
\[\frac{16}{2}\Omega \]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 16) In a parallel, plate capacitor of capacitance C, a metal sheet is inserted between the plates, parallel to them. If the thickness of the sheet is reduced to half of the separation between the plates. The capacitance will be:
A)
\[\frac{C}{2}\]
done
clear
B)
\[\frac{3C}{4}\]
done
clear
C)
\[4C\]
done
clear
D)
\[2C\]
done
clear
View Answer play_arrow
question_answer 17) The forward voltage in a diode increased the width of the depletion region:
A)
does not change
done
clear
B)
decreases
done
clear
C)
increases
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 18) An electric bulb is designed to draw power \[{{P}_{0}}\]at voltage \[{{V}_{0}}.\]If the voltage is V it draws a power\[{{P}_{2}}.\]Then:
A)
\[P={{\left( \frac{{{V}_{0}}}{V} \right)}^{2}}{{P}_{0}}\]
done
clear
B)
\[P={{\left( \frac{V}{{{V}_{0}}} \right)}^{2}}{{P}_{0}}\]
done
clear
C)
\[P=\left( \frac{V}{{{V}_{0}}} \right){{P}_{0}}\]
done
clear
D)
\[P=\left( \frac{{{V}_{0}}}{V} \right){{P}_{0}}\]
done
clear
View Answer play_arrow
question_answer 19) A magnet dropped into a coil of conducting wire along its axis will fall with an acceleration:
A)
more than g
done
clear
B)
equal to g
done
clear
C)
less than g
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 20) The resistors of resistances \[2\Omega ,4\Omega \]and \[8\Omega \] are connected in parallel, then the equivalent resistance of the combination will be:
A)
\[\frac{8}{7}\Omega \]
done
clear
B)
\[\frac{7}{8}\Omega \]
done
clear
C)
\[\frac{7}{4}\Omega \]
done
clear
D)
\[\frac{4}{9}\Omega \]
done
clear
View Answer play_arrow
question_answer 21) A ball of mass\[1g\]and charge \[{{10}^{-8}}C\]moves from a point A. Where potential is 600 volt to the point B where potential is zero. Velocity of the ball at the point B is 20 cm/s. The velocity of the ball at the point A will be:
A)
\[~22.8\text{ }cm/s\]
done
clear
B)
\[~228\text{ }cm/s\]
done
clear
C)
\[16.8\text{ }m/s\]
done
clear
D)
\[~168\text{ }m/s\]
done
clear
View Answer play_arrow
question_answer 22) A small object is placed 10 cm in front of a plane mirror. If you stand behind the object 30 cm from the mirror and look at its image, the distance recused for your eye will be:
A)
60 cm
done
clear
B)
20 cm
done
clear
C)
40 cm
done
clear
D)
80 cm
done
clear
View Answer play_arrow
question_answer 23) A milliammeter of range 10 mA has a coil of resistance \[1\Omega .\] To use it as voltmeter of range 10 volt, the resistance that must be connected in series with it, will be:
A)
\[999\,\Omega \]
done
clear
B)
\[99\,\Omega \]
done
clear
C)
\[~1000\text{ }\Omega \]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 24) While a capacitor remains connected to a battery and dielectric slab is applied between the plates, then:
A)
potential difference between the plates is changed
done
clear
B)
charge flows from the battery to the capacitor
done
clear
C)
electric field between the plates increases
done
clear
D)
energy store in the capacitor Decreases
done
clear
View Answer play_arrow
question_answer 25) There are two electric bulbs of 40 volt and 60 watt. The ratio of their resistance will be:
A)
5:4
done
clear
B)
4 : 3
done
clear
C)
3 : 2
done
clear
D)
2 : 3
done
clear
View Answer play_arrow
question_answer 26) A charge \[q\]is placed at the centre of the line joining two equal charges Q. The system of there charges will be in equilibrium if q is equal to:
A)
\[-\frac{Q}{4}\]
done
clear
B)
\[-\frac{Q}{2}\]
done
clear
C)
\[+\frac{Q}{2}\]
done
clear
D)
\[+\frac{Q}{4}\]
done
clear
View Answer play_arrow
question_answer 27) If a ball is thrown vertically upwards; assuming the air resistance to be constant and considerable, then:
A)
time of ascent \[\ge \] time of descent
done
clear
B)
time of ascent \[=\] time of descent
done
clear
C)
time of ascent < time of descent
done
clear
D)
time of ascent > time of descent
done
clear
View Answer play_arrow
question_answer 28) The work function for aluminium is 4.125 eV. The cutoff wavelength for photoelectric effect for aluminium will be:
A)
420 nm
done
clear
B)
350 nm
done
clear
C)
300 nm
done
clear
D)
200 nm
done
clear
View Answer play_arrow
question_answer 29) A bus is moving with a velocity of 5 m/s towards a huge well. The driver sounds a horn of frequency 165 Hz. If the speed of sound in air is 355 m/s, the number of beats heard per second by a passenger on the bus will be:
A)
6
done
clear
B)
5
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 30) If the critical angle for total internal reflection from a medium to vacuum is\[{{30}^{o}},\]then velocity of light in the medium is:
A)
\[1.5\times {{10}^{8}}m/s\]
done
clear
B)
\[3\times {{10}^{8}}m/s\]
done
clear
C)
\[0.75\,m/s\]
done
clear
D)
\[6\times {{10}^{8}}\,m/s\]
done
clear
View Answer play_arrow
question_answer 31) In a stationary wave, all particles of the medium cross the mean position with:
A)
different velocities at different instants
done
clear
B)
different velocities at same instants
done
clear
C)
same speed at all instants
done
clear
D)
different speed at all instants
done
clear
View Answer play_arrow
question_answer 32) A cylinder of 5 litre capacity, filled with air at N.T.P. is connected with another evacuated cylinder of 30 litres of capacity the resultant air pressure in both the cylinders will be:
A)
\[38.85\text{ }cm\]of Hg
done
clear
B)
\[21.85\text{ }cm\]of Hg
done
clear
C)
\[10.85\text{ }cm\] of Hg
done
clear
D)
\[~14.85\text{ }cm\] of Hg
done
clear
View Answer play_arrow
question_answer 33) As the intensity of incident light increases then:
A)
K.E. of photoelectron decreases
done
clear
B)
K.E. of photoelectron increases
done
clear
C)
photoelectric current increases
done
clear
D)
photoelectric current decreases
done
clear
View Answer play_arrow
question_answer 34) Which of the following statement is not the true?
A)
while taking reading of tangent galvanometer, the plane of the coil must be set at right angles to the earth's magnetic meridian
done
clear
B)
a short magnet is used in a tangent galvanometer since a long magnet would be heavy and may not easily move
done
clear
C)
measurements with the tangent galvanometer will be more accurate, when the deflection is around \[{{45}^{o}}\]
done
clear
D)
a tangent galvanometer can not be used in the polar region
done
clear
View Answer play_arrow
question_answer 35) Pick out the statement which is not true:
A)
shortest wavelength UV radiation are beneficial to living tissue while longer wavelength are harmful:
done
clear
B)
UV radiation have wavelength extending from 200 nm to 400 nm.
done
clear
C)
UV radiation are used for sterlisation of water
done
clear
D)
sun is natural source of UV radiation
done
clear
View Answer play_arrow
question_answer 36) The relative permeability is represented by \[{{\mu }_{r}}\]and the susceptibility is denoted by \[\chi \]for a magnetic substance. Then for a paramagnetic substance:
A)
\[{{\mu }_{r}}<1,\chi <0\]
done
clear
B)
\[{{\mu }_{r}}<1,\chi >0\]
done
clear
C)
\[{{\mu }_{r}}>1,\chi <0\]
done
clear
D)
\[{{\mu }_{r}}>1,\chi >0\]
done
clear
View Answer play_arrow
question_answer 37) An electron moving in a circular orbit of radius \[r\]makes \[n\]rotation per second. The magnetic field produced at the centre has a magnitude of:
A)
\[\frac{{{\mu }_{0}}ne}{2r}\]
done
clear
B)
\[\frac{{{\mu }_{0}}{{n}^{2}}e}{2r}\]
done
clear
C)
\[\frac{{{\mu }_{0}}ne}{2\pi r}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 38) A train is moving towards north with a speed of 180 km/hr. If the vertical component of the earth's magnetic field is \[0.2\times {{10}^{-4}}T,\]the e .m. f. induced, in the axle 1-5m long, is:
A)
1.5 mV
done
clear
B)
15 mV
done
clear
C)
5.4 mV
done
clear
D)
54 mV
done
clear
View Answer play_arrow
question_answer 39) When a ceiling fan is switched off its angular velocity radius 50% while its makes 36 rotations. How many more rotation will it make before coming to rest (Assume uniform angular retardation):
A)
18
done
clear
B)
12
done
clear
C)
36
done
clear
D)
48
done
clear
View Answer play_arrow
question_answer 40) During an adiabatic process the pressure of a gas is found to be proportional to the cube of its absolute temperature. The ratio of \[{{C}_{p}}/{{C}_{\upsilon }}\]for the gas is:
A)
\[\frac{3}{2}\]
done
clear
B)
\[\frac{4}{3}\]
done
clear
C)
2
done
clear
D)
\[\frac{5}{3}\]
done
clear
View Answer play_arrow
question_answer 41) Three fourth of the active nuclei present in a radioactive sample decay in 3/4 sec. The half life of the sample is:
A)
\[\frac{1}{2}\sec \]
done
clear
B)
\[1\,\sec \]
done
clear
C)
\[\frac{3}{8}\sec \]
done
clear
D)
\[\frac{3}{4}\sec \]
done
clear
View Answer play_arrow
question_answer 42) A gas at the temperature 250 K contained in a closed vessel. If the gas is heated through 1 K, percentage increase in its pressure will be:
A)
\[0.4%\]
done
clear
B)
\[0.2%\]
done
clear
C)
\[0.1%\]
done
clear
D)
\[0.8%\]
done
clear
View Answer play_arrow
question_answer 43) Transverse waves of same frequency are generated in two steel wires A and B. The diameter of A is twice of B and the tension in A is half that in B. The ratio of velocities of wave in A and B is:
A)
\[1:3\sqrt{2}\]
done
clear
B)
\[1:2\sqrt{2}\]
done
clear
C)
\[1:2\]
done
clear
D)
\[\sqrt{2}:1\]
done
clear
View Answer play_arrow
question_answer 44) A block of mass 1kg slide down a rough inclined plane of inclination \[{{60}^{o}}\]starting from its top. If coefficient of kinetic friction is 0.5 and length of the plane\[d=1\text{ }m\]then work done against friction is:
A)
2.45 J
done
clear
B)
4.9J
done
clear
C)
9.8 J
done
clear
D)
19.6J
done
clear
View Answer play_arrow
question_answer 45) A bar magnet of magnetic M is cut into two parts of equal length. The magnetic moment of each part will be
A)
12M
done
clear
B)
M
done
clear
C)
0.5M
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 46) A body, thrown upwards with some velocity, reaches the maximum height of 20 m. Another body with double the mass thrown up, with double initial velocity will reach a maximum of:
A)
200 m
done
clear
B)
16 am
done
clear
C)
80 m
done
clear
D)
40 m
done
clear
View Answer play_arrow
question_answer 47) A particle having charge 100 times that of an electron is revolving in a circular path of radius 0-8 m with one rotation per second. Magnetic field produced at the centre of particle will be:
A)
\[{{10}^{-17}}{{\mu }_{0}}\]
done
clear
B)
\[{{10}^{-11}}{{\mu }_{0}}\]
done
clear
C)
\[{{10}^{-7}}{{\mu }_{0}}\]
done
clear
D)
\[{{10}^{-3}}{{\mu }_{0}}\]
done
clear
View Answer play_arrow
question_answer 48) The resultant of two forces 3P and IP is R If the first force is doubled then the resultant is also doubled. The angle between the two forces is:
A)
\[{{60}^{o}}\]
done
clear
B)
\[{{120}^{o}}\]
done
clear
C)
\[{{70}^{o}}\]
done
clear
D)
\[{{180}^{o}}\]
done
clear
View Answer play_arrow
question_answer 49) In an\[~n-p-n\] transistor circuit, the collector current is 10 mA. If 90% of the electrons emitted reach the collector, the emitter current je and base current \[IE\]are given by:
A)
\[{{I}_{E}}=9\,mA,\,{{I}_{B}}=-1\,mA\]
done
clear
B)
\[{{I}_{E}}=-1\,mA,\,{{I}_{B}}=9\,mA\]
done
clear
C)
\[{{I}_{E}}=1\,mA,\,{{I}_{B}}=11\,mA\]
done
clear
D)
\[{{I}_{E}}=11\,mA,{{I}_{B}}=1\,mA\]
done
clear
View Answer play_arrow
question_answer 50) The energy of hydrogen atom in its ground state is \[-13.6\text{ }eV.\] The energy of the level corresponding to the quantum number\[n\]is equal 5 is:
A)
\[-5.40\,eV\]
done
clear
B)
\[~-2.72\,eV\]
done
clear
C)
\[~-\text{ }0.85\text{ }eV\]
done
clear
D)
\[~-\text{ }0.54\text{ }eV\]
done
clear
View Answer play_arrow
question_answer 51) Which of the following relation is correct in magnetism?
A)
\[{{V}^{2}}=I+H\]
done
clear
B)
\[V={{I}^{2}}+{{H}^{2}}\]
done
clear
C)
\[I=V+H\]
done
clear
D)
\[{{I}^{2}}={{V}^{2}}+{{H}^{2}}\]
done
clear
View Answer play_arrow
question_answer 52) Magnetic fields at two points on the axis of a circular coil at a distance of 0.05 m and 0.2 m from the1 centre are in the ratio 8:1. The radius of the coil is:
A)
\[1.0\,m\]
done
clear
B)
\[0.1\,m\]
done
clear
C)
\[0.15\,m\]
done
clear
D)
\[0.2\,m\]
done
clear
View Answer play_arrow
question_answer 53) A balloon starts rising from the ground with an acceleration of \[1.25\,m/{{s}^{2}}\]after 8s, a stone is released from the balloon. The stone will \[(g=10\,m/{{s}^{2}})\]:
A)
reach the ground in 4 second
done
clear
B)
begin to move down after being released
done
clear
C)
have a displacement of 50 m
done
clear
D)
cover a distance of 40 m in reaching the ground
done
clear
View Answer play_arrow
question_answer 54) If a white light is used in Young's double slit experiments then a very large number of coloured fringes can be seen:
A)
with first order violet fringes being closer to the central white fringes
done
clear
B)
first order red fringes being closer to the central white fringes
done
clear
C)
with a central white fringe
done
clear
D)
with a central black fringe
done
clear
View Answer play_arrow
question_answer 55) In the experiment of diffraction at a single slit, if the slit width is decreased, the width of the central maximum:
A)
decreases in Fresnel?s diffraction but increases in Fraun hofer diffraction
done
clear
B)
increases in Fresnel?s diffraction but decreases in Fraun hofer diffraction
done
clear
C)
decreases in both Fresnel and Fraun hofer diffraction
done
clear
D)
increases in both Fresnel and Fraun hoofer diffraction
done
clear
View Answer play_arrow
question_answer 56) A satellite with kinetic energy\[{{E}_{k}}\]is revolving round the earth in a circular orbit. How much more kinetic energy should be given to it so that it may just escape into outer space.
A)
\[{{E}_{k}}\]
done
clear
B)
\[2{{E}_{k}}\]
done
clear
C)
\[\frac{1}{2}{{E}_{k}}\]
done
clear
D)
\[3{{E}_{k}}\]
done
clear
View Answer play_arrow
question_answer 57) The ionisation potential of hydrogen is \[13.6\text{ }eV.\] Then the energy released when an electron jumps from \[n=3\]to \[n=2\]orbit, is:
A)
\[2.89\,eV\]
done
clear
B)
\[1.89\,eV\]
done
clear
C)
\[3.89\,eV\]
done
clear
D)
\[4.89\,eV\]
done
clear
View Answer play_arrow
question_answer 58) In an X-ray tube electrons bombarding the target produce X-rays of minimum wavelength \[1\overset{\text{o}}{\mathop{\text{A}}}\,.\]What must be the energy of bombarding electrons:
A)
13375 eV
done
clear
B)
12375 eV
done
clear
C)
14375 eV
done
clear
D)
15375 eV
done
clear
View Answer play_arrow
question_answer 59) Two progressive wave are represented by the following equations: \[{{y}_{1}}=5\sin 2\pi (10t-0.1x)\] \[{{y}_{2}}=10\sin 2\pi (20\,t-0.2x)\] The ratio of intensities will be:
A)
1 : 16
done
clear
B)
1 : 4
done
clear
C)
1 : 2
done
clear
D)
1 : 1
done
clear
View Answer play_arrow
question_answer 60) The frequency of a tuning fork is 384 per second and velocity of sound in air is 352 m/s. How far the sound has traversed while fork completes 36 vibration?
A)
3 m
done
clear
B)
13 m
done
clear
C)
23 m
done
clear
D)
33m
done
clear
View Answer play_arrow
question_answer 61) At what temperature will the RMS velocity of \[\text{S}{{\text{O}}_{\text{2}}}\]be the same as that of \[{{\text{O}}_{\text{2}}}\] at\[303\text{ }K\]?
A)
403K
done
clear
B)
303K
done
clear
C)
606 K
done
clear
D)
273 K
done
clear
View Answer play_arrow
question_answer 62) Phenol is treated with bromine water and shaken well. The white precipitate formed during the process is:
A)
\[m-\]bromophenol
done
clear
B)
2, 4, 6 tribromophenol
done
clear
C)
\[2-4\]dibromophenol
done
clear
D)
a mixture of o-and \[p-\]bromophenols
done
clear
View Answer play_arrow
question_answer 63) In thermite process the metal used as reducing agent is:
A)
nickel
done
clear
B)
zinc
done
clear
C)
sodium
done
clear
D)
aluminium
done
clear
View Answer play_arrow
question_answer 64) Which of the following compounds on boiling with alkaline \[\text{KMn}{{\text{O}}_{\text{4}}}\]and subsequent acidification will not give benzoic acid?
A)
benzyl alcohol
done
clear
B)
acetophenone
done
clear
C)
anisole
done
clear
D)
toluene
done
clear
View Answer play_arrow
question_answer 65) The base present in DNA, but not in RNA is:
A)
guanine
done
clear
B)
adenine
done
clear
C)
uracil
done
clear
D)
thymine
done
clear
View Answer play_arrow
question_answer 66) The total number of lattice arrangements in different crystal system is:
A)
7
done
clear
B)
3
done
clear
C)
8
done
clear
D)
14
done
clear
View Answer play_arrow
question_answer 67) The conversion of A to B follows second order kinetics. Doubling the concentration of A will increase the rate of formation of B by a factor of:
A)
4
done
clear
B)
2
done
clear
C)
1/4
done
clear
D)
½
done
clear
View Answer play_arrow
question_answer 68) \[R-COO{{R}^{1}}+{{H}_{2}}O\xrightarrow{HCl}\]\[R-COOH+{{R}^{1}}OH.\] What type of reaction is this?
A)
second order
done
clear
B)
unimolecular
done
clear
C)
pseudo-unimolecular
done
clear
D)
third order
done
clear
View Answer play_arrow
question_answer 69) The unit of quantity of electricity is:
A)
ohm
done
clear
B)
ampere
done
clear
C)
coulomb
done
clear
D)
volt
done
clear
View Answer play_arrow
question_answer 70) \[p{{K}_{a}}\]values of two acids A and B are 4 and 5. The strengths of these two acids are related as:
A)
acid A is 10 times stronger than acid B
done
clear
B)
strength of acid A : strengths of acid \[B=4:5\]
done
clear
C)
the strengths of the two acids can not be compared
done
clear
D)
acid B is 10 times stronger than acid A
done
clear
View Answer play_arrow
question_answer 71) \[\text{10}\,\text{d}{{\text{m}}^{\text{2}}}\]of \[{{\text{N}}_{2}}\]gas and \[\text{10}\,\text{d}{{\text{m}}^{3}}\]of gas X at the same temperature and pressure contain the same number of molecules The gas X is:
A)
\[NO\]
done
clear
B)
\[{{H}_{2}}\]
done
clear
C)
\[C{{O}_{2}}\]or \[Co\]
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 72) When the electrons of hydrogen atoms return to L shell from shells of higher energy, we get a series of lines in the spectrum. This series is called:
A)
Balmer series
done
clear
B)
Lyman series
done
clear
C)
Bracket! Series
done
clear
D)
Paschen series
done
clear
View Answer play_arrow
question_answer 73) The alcohol obtained by the hydrolysis of oils and fats is:
A)
glycol
done
clear
B)
glycerol
done
clear
C)
propanol
done
clear
D)
pentanol
done
clear
View Answer play_arrow
question_answer 74) Which of the following alloys is used for making magnets for hearing aids?
A)
ainico
done
clear
B)
german silver
done
clear
C)
invar
done
clear
D)
monel metal
done
clear
View Answer play_arrow
question_answer 75) One \[\text{d}{{\text{m}}^{\text{3}}}\]of 2M ethanoic acid is mixed with one \[\text{d}{{\text{m}}^{\text{3}}}\]of 3M ethanol to form an ester \[C{{H}_{3}}COOH+{{C}_{2}}{{H}_{5}}OH\xrightarrow{{}}\]\[C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}+{{H}_{2}}O\] The decrease in the initial rate if each solution is diluted with an equal volume of water would be:
A)
2 times
done
clear
B)
4 times
done
clear
C)
0.25 times
done
clear
D)
0.5 times
done
clear
View Answer play_arrow
question_answer 76) Which one of the following can be classified as a Bronstead base?
A)
\[NO_{3}^{-}\]
done
clear
B)
\[{{H}_{3}}{{O}^{+}}\]
done
clear
C)
\[C{{H}_{3}}COOH\]
done
clear
D)
\[NH_{4}^{+}\]
done
clear
View Answer play_arrow
question_answer 77) pH of a solution produced when an aqueous solution of pH 6 is mixed with an equal volume of an aqueous solution of pH 3 is about:
A)
3.3
done
clear
B)
4.3
done
clear
C)
4.0
done
clear
D)
4.5
done
clear
View Answer play_arrow
question_answer 78) Aluminium displaces hydrogen from acids, but copper does not. A Galvanic cell prepared by combining \[\text{Cu/C}{{\text{u}}^{\text{2}}}\text{+}\]and \[\text{Al/A}{{\text{l}}^{\text{3+}}}\]has an emf of\[\text{2}\text{.0 V}\] at\[\text{298 K}\text{.}\]If the potential of copper electrode is\[\text{+ 0}\text{.34 V,}\] that of aluminium electrode is:
A)
\[-\,2.3V\]
done
clear
B)
\[+\,2.34V\]
done
clear
C)
\[-1.66\,V\]
done
clear
D)
\[+\,1.66\,V\]
done
clear
View Answer play_arrow
question_answer 79) 1.5 moles of \[{{\text{O}}_{\text{2}}}\] combine with Mg to form the oxide \[\text{MgO}\text{.}\]The mass of Mg that has combined is \[\text{(Mg}\,\text{=}\,\text{24)}\,\text{:}\]
A)
72 g
done
clear
B)
36 g
done
clear
C)
48 g
done
clear
D)
24 g
done
clear
View Answer play_arrow
question_answer 80) How much of\[\text{NaOH}\]is required to neutralise \[\text{1500}\,\text{c}{{\text{m}}^{\text{3}}}\] of \[\text{0}\text{.1}\,\text{N}\,\text{HCl?}\]\[\text{(Na}\,\text{=}\,\text{23):}\]
A)
60 g
done
clear
B)
6 g
done
clear
C)
4 g
done
clear
D)
40 g
done
clear
View Answer play_arrow
question_answer 81) The boiling point of water is exceptionally high because:
A)
water molecule is not linear
done
clear
B)
water molecule is linear
done
clear
C)
there is covalent bond between H and O
done
clear
D)
water molecules associate due to hydrogen bonding
done
clear
View Answer play_arrow
question_answer 82) \[{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{10}}}\]is not used with to dry \[\text{N}{{\text{H}}_{\text{3}}}\]gas because:
A)
\[{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{10}}}\]is basic and \[\text{N}{{\text{H}}_{3}}\]is acidic
done
clear
B)
\[{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{10}}}\]is acidic and\[\text{N}{{\text{H}}_{3}}\] is basic
done
clear
C)
\[{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{10}}}\]is not a drying agent
done
clear
D)
\[{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{10}}}\] reacts with moisture in\[\text{N}{{\text{H}}_{\text{3}}}\]
done
clear
View Answer play_arrow
question_answer 83) A radioactive isotope has a half life of 10 years. What percentage of the original amount of it remain after 20 years:
A)
0
done
clear
B)
12.5
done
clear
C)
8
done
clear
D)
25
done
clear
View Answer play_arrow
question_answer 84) 75% of a first order reaction is completed in 30 minutes. What is the time required for 93.75% of the reaction (in minutes)?
A)
45
done
clear
B)
120
done
clear
C)
90
done
clear
D)
60
done
clear
View Answer play_arrow
question_answer 85) Excess of \[\text{N}{{\text{a}}^{\text{+}}}\] ions in our system causes:
A)
high B.P.
done
clear
B)
low B.P.
done
clear
C)
diabetes
done
clear
D)
anaemia
done
clear
View Answer play_arrow
question_answer 86) Alcoholic potash is used to bring about:
A)
dehydrogenation
done
clear
B)
dehydration
done
clear
C)
dehydro halogenation
done
clear
D)
dehalogenation
done
clear
View Answer play_arrow
question_answer 87) Which of the following organic compounds answers both iodoform test and Fehling's test?
A)
methanol
done
clear
B)
ethanol
done
clear
C)
propane
done
clear
D)
ethanol
done
clear
View Answer play_arrow
question_answer 88) Gold sol is an electronegative sol. The amount of electrolyte required to coagulate a certain amount of gold sol is minimum in the case of:
A)
\[CaC{{l}_{2}}\]
done
clear
B)
\[NaCl\]
done
clear
C)
\[AlC{{l}_{3}}\]
done
clear
D)
\[N{{a}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 89) The oxidation number and the electronic configuration of sulphur in \[{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\]is:
A)
\[+\,4;\,1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}\]
done
clear
B)
\[+\,2;\,1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{2}}\]
done
clear
C)
\[+\,3;\,1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{1}}\]
done
clear
D)
\[+\,6;\,1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}\]
done
clear
View Answer play_arrow
question_answer 90) The mass of \[\text{112}\,\text{c}{{\text{m}}^{\text{3}}}\]of \[\text{C}{{\text{H}}_{\text{4}}}\] gas at STP is:
A)
0.16 g
done
clear
B)
0.8 g
done
clear
C)
0.08 g
done
clear
D)
\[1.6\,g\]
done
clear
View Answer play_arrow
question_answer 91) The volume of water to be added to \[\text{100 c}{{\text{m}}^{\text{3}}}\]of \[\text{0}\text{.5}\,\text{N}\,{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\]to get decinormal concentration is:
A)
\[400\text{ }c{{m}^{3}}\]
done
clear
B)
\[~500\text{ }c{{m}^{3}}\]
done
clear
C)
\[~450\text{ }c{{m}^{3}}\]
done
clear
D)
\[~100\text{ }c{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 92) In which of the following equilibrium systems is the rate of the backward reaction favoured by increase of pressure?
A)
\[PC{{l}_{5}}\rightleftharpoons PC{{l}_{3}}+C{{l}_{2}}\]
done
clear
B)
\[2S{{O}_{2}}+{{O}_{2}}\rightleftharpoons 2N{{H}_{3}}\]
done
clear
C)
\[{{N}_{2}}+3{{H}_{2}}\rightleftharpoons 2N{{H}_{3}}\]
done
clear
D)
\[{{N}_{2}}+{{O}_{2}}\rightleftharpoons 2NO\]
done
clear
View Answer play_arrow
question_answer 93) In order to decompose 9g water \[\text{142}\text{.5 kJ}\]heat is required. Hence the enthalpy of formation of water is:
A)
\[-142.5\,\,kJ\]
done
clear
B)
\[+\,142.5\,kJ\]
done
clear
C)
\[-285\,kJ\]
done
clear
D)
\[+\,285\,kJ\]
done
clear
View Answer play_arrow
question_answer 94) A quantity of \[\text{PC}{{\text{l}}_{\text{5}}}\]was heated in a \[\text{10 d}{{\text{m}}^{\text{3}}}\]vessel at \[\text{250}{{\,}^{\text{o}}}\text{C}\]\[\text{PC}{{\text{l}}_{5}}(g)\rightleftharpoons PC{{l}_{3}}(g)C{{l}_{2}}(g)\] At equilibrium the vessel contains\[0.1\]mole of \[\text{PC}{{\text{l}}_{\text{5}}}\]and\[0.2\]mole of \[\text{C}{{\text{l}}_{\text{2}}}\text{.}\] The equilibrium constant of the reaction is:
A)
\[0.05\]
done
clear
B)
\[0.02\]
done
clear
C)
\[0.025\]
done
clear
D)
\[0.04\]
done
clear
View Answer play_arrow
question_answer 95) Which among the following species has the highest ionisation potential?
A)
B
done
clear
B)
Li
done
clear
C)
Ne
done
clear
D)
F
done
clear
View Answer play_arrow
question_answer 96) Which one of the following metallic hydroxides does not dissolve in sodium hydroxide solution?
A)
\[Zn{{(OH)}_{2}}\]
done
clear
B)
\[Al{{(OH)}_{3}}\]
done
clear
C)
\[Fe{{(OH)}_{3}}\]
done
clear
D)
\[Pb{{(OH)}_{2}}\]
done
clear
View Answer play_arrow
question_answer 97) IUPAC name of \[{{\text{K}}_{\text{3}}}\text{Fe(CN}{{\text{)}}_{\text{6}}}\]is:
A)
potassium ferricyanide
done
clear
B)
hexacyano ferrate (III)
done
clear
C)
potassium hexacyano ferrate (III)
done
clear
D)
potassium hexacyano ferrate (II)
done
clear
View Answer play_arrow
question_answer 98) Unpleasant smell of carbylamine is obtained when chloroform and alcoholic KOH are heated with:
A)
any aliphatic amine
done
clear
B)
any amine
done
clear
C)
any primary amine
done
clear
D)
any aromatic amine
done
clear
View Answer play_arrow
question_answer 99) Which of the following is an aldohexose?
A)
cellulose
done
clear
B)
sucrose
done
clear
C)
glucose
done
clear
D)
raffinose
done
clear
View Answer play_arrow
question_answer 100) When a mixture of calcium acetate and calcium formate is dry distilled, the product formed as:
A)
ethanal
done
clear
B)
butanone
done
clear
C)
methanal
done
clear
D)
acetophenone
done
clear
View Answer play_arrow
question_answer 101) The test used for identifying peptide linkage in proteins is:
A)
Borsche's test
done
clear
B)
Molisch's test
done
clear
C)
Ninhydrin test
done
clear
D)
Biuret test
done
clear
View Answer play_arrow
question_answer 102) Which of the following is not used to distinguish ethene from ethane?
A)
iodine in \[\text{CC}{{\text{l}}_{4}}\]
done
clear
B)
bromine in\[\text{CC}{{\text{l}}_{4}}\]
done
clear
C)
alkaline \[\text{KMn}{{\text{O}}_{\text{4}}}\]
done
clear
D)
ammonical \[\text{C}{{\text{u}}_{\text{2}}}\text{C}{{\text{l}}_{\text{2}}}\]
done
clear
View Answer play_arrow
question_answer 103) To get a n-type doped semiconductor, impurity to be added to silicon should have the following number of valence electrons:
A)
1
done
clear
B)
3
done
clear
C)
5
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 104) For the equilibrium\[{{N}_{2}}(g)+3{{H}_{2}}(g)\rightleftharpoons 2N{{H}_{3}}(g),\] The equilibrium constant,\[{{K}_{p}}\]is equal to:
A)
\[{{K}_{c}}={{(RT)}^{2}}\]
done
clear
B)
\[{{K}_{c}}(RT)\]
done
clear
C)
\[\frac{{{K}_{c}}}{{{(RT)}^{2}}}\]
done
clear
D)
\[{{K}_{c}}\]
done
clear
View Answer play_arrow
question_answer 105) 5 moles of \[\text{S}{{\text{O}}_{\text{2}}}\]and 5 moles of \[{{\text{O}}_{2}}\] are allowed to react to form\[\text{S}{{\text{O}}_{\text{3}}}\]in a closed vessel. At the equilibrium stage 60% of \[\text{S}{{\text{O}}_{2}}\]is used up. The total number of moles of\[\text{S}{{\text{O}}_{\text{2}}}\text{,}{{\text{O}}_{\text{2}}}\]and\[\text{S}{{\text{O}}_{\text{3}}}\]in the vessel now is:
A)
\[10.0\]
done
clear
B)
\[8.5\]
done
clear
C)
\[10.5\]
done
clear
D)
\[3.9\]
done
clear
View Answer play_arrow
question_answer 106) For a given reaction\[{{t}_{1/2}}=\frac{1}{Ka}.\]The Ka order of the reaction is:
A)
1
done
clear
B)
0
done
clear
C)
3
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 107) In the case of small cuts, bleeding is stopped by applying potash alum. Here alum acts as:
A)
fungicide
done
clear
B)
disinfectant
done
clear
C)
germicide
done
clear
D)
coagulating agent
done
clear
View Answer play_arrow
question_answer 108) For the reaction\[{{H}_{2}}O(s)\rightleftharpoons {{H}_{2}}O(l)\]at \[0{{\,}^{o}}C\]and normal pressure :
A)
\[\Delta H >T \Delta \,S\]
done
clear
B)
\[\Delta H =T \Delta \,S\]
done
clear
C)
\[\Delta H =\Delta \,G\]
done
clear
D)
\[\Delta H <T\Delta \,S\]
done
clear
View Answer play_arrow
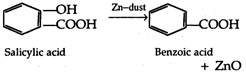
question_answer 109) When salicylic acid is distilled with zinc dust, the product obtained is:
A)
zinc salicylate
done
clear
B)
salicylaldehyde
done
clear
C)
phenol
done
clear
D)
benzoic acid
done
clear
View Answer play_arrow
question_answer 110)
A)
28
done
clear
B)
18
done
clear
C)
85
done
clear
D)
46
done
clear
View Answer play_arrow
question_answer 111) On heating ethanol with excess of cone. \[{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\]at \[\text{170}{{\,}^{\text{o}}}\text{C,}\] the product obtained is:
A)
ethene
done
clear
B)
ethoxy ethane
done
clear
C)
ethyne
done
clear
D)
ethane
done
clear
View Answer play_arrow
question_answer 112) To calculate the amount of work done in joules during reversible isothermal expansion of an ideal gas, the volume must be expressed in:
A)
\[d{{m}^{3}}\]only
done
clear
B)
\[{{m}^{3}}\]only
done
clear
C)
any unit
done
clear
D)
\[c{{m}^{3}}\]unit
done
clear
View Answer play_arrow
question_answer 113) The quantity of electricity required to liberate \[\text{112 c}{{\text{m}}^{\text{3}}}\]of hydrogen at STP from acidified water is:
A)
965 Coulombs
done
clear
B)
1 Faraday
done
clear
C)
0.1 Faraday
done
clear
D)
96500 Coulombs
done
clear
View Answer play_arrow
question_answer 114) Hydrogen ion concentration of an aqueous solution is \[1\times {{10}^{-4}}M.\]The solution is diluted with equal volume of water. Hydroxyl ion concentration of the resultant solution in terms of mol \[d{{m}^{-3}}\]is:
A)
\[1\times {{10}^{-8}}\]
done
clear
B)
\[1\times {{10}^{-6}}\]
done
clear
C)
\[2\times {{10}^{-10}}\]
done
clear
D)
\[0.5\times {{10}^{-10}}\]
done
clear
View Answer play_arrow
question_answer 115) The shape of cuprammonium ion is:
A)
trigonal
done
clear
B)
tetrahedral
done
clear
C)
octahedral
done
clear
D)
square planar
done
clear
View Answer play_arrow
question_answer 116) The enthalpy of combustion of \[{{C}_{6}}{{H}_{6}}(l)\]is \[-3250\,kJ.\]When \[\text{0}\text{.39 g}\]of benzene is burnt in excess of oxygen in an open vessel, the amount of heat liberated is:
A)
\[\text{16}\text{.25}\,\text{J}\]
done
clear
B)
\[\text{16}\text{.25}\,k\text{J}\]
done
clear
C)
\[32.5\,J\]
done
clear
D)
\[32.5\,kJ\]
done
clear
View Answer play_arrow
question_answer 117) In an adiabatic expansion of an ideal gas:
A)
\[W=-\Delta E\]
done
clear
B)
\[W=\Delta E\]
done
clear
C)
\[\Delta E=0\]
done
clear
D)
\[W=0\]
done
clear
View Answer play_arrow
question_answer 118) The solubility product of a binary weak electrolyte is \[4\times {{10}^{-10}}\,\]at 298 K. Its solubility in \[\text{mol}\,\text{d}{{\text{m}}^{-3}}\]at the same temperature is:
A)
\[4\times {{10}^{-5}}\]
done
clear
B)
\[2\times {{10}^{-5}}\]
done
clear
C)
\[8\times {{10}^{-10}}\]
done
clear
D)
\[16\times {{10}^{-20}}\]
done
clear
View Answer play_arrow
question_answer 119) Propane is the product obtained by dehydrogenation of:
A)
2-propanol
done
clear
B)
1-propanol
done
clear
C)
propanal
done
clear
D)
\[n-\]propyl alcohol
done
clear
View Answer play_arrow
question_answer 120) To get DDT chlorobenzene has to react with the following compound in the pressure of concentrated sulphuric acid:
A)
trichloro ethane
done
clear
B)
dichloro acetone
done
clear
C)
dichloro acetaldehyde
done
clear
D)
trichloro acetaldehyde
done
clear
View Answer play_arrow
question_answer 121) If \[{{\,}^{n}}{{P}_{4}}=24{{\,}^{n}}{{C}_{5}},\]then the value of \[n:\]
A)
10
done
clear
B)
15
done
clear
C)
9
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 122) The angle between the lines\[{{x}^{2}}+4xy+{{y}^{2}}=0\]
A)
\[~{{60}^{o}}\]
done
clear
B)
\[{{15}^{o}}\]
done
clear
C)
\[~{{30}^{o}}\]
done
clear
D)
\[~{{45}^{o}}\]
done
clear
View Answer play_arrow
question_answer 123) Separate equations of lines, whose equation is \[{{x}^{2}}+xy-12{{y}^{2}}=0\]are:
A)
\[x+4y=0\]and\[x+3y=0\]
done
clear
B)
\[2x-3y=0\]and \[x-4y=0\]
done
clear
C)
\[x-6y=0\]and \[x-3y=0\]
done
clear
D)
\[x+4y=0\]and \[x-3y=0\]
done
clear
View Answer play_arrow
question_answer 124) The value of\[\underset{x\to 0}{\mathop{\lim }}\,\left( \frac{{{e}^{x}}-1}{x} \right)\]is:
A)
\[1/2\]
done
clear
B)
\[\infty \]
done
clear
C)
1
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 125) The value of\[\underset{x\to 0}{\mathop{\lim }}\,\left[ \frac{\sqrt{a+x}-\sqrt{a-x}}{x} \right]\]is:
A)
1
done
clear
B)
zero
done
clear
C)
\[\sqrt{a}\]
done
clear
D)
\[\frac{1}{\sqrt{a}}\]
done
clear
View Answer play_arrow
question_answer 126) If \[A=\left[ \begin{matrix} 2 & 3 \\ 4 & 6 \\ \end{matrix} \right],\]then \[{{A}^{-1}}\]is equal to:
A)
\[\left[ \begin{matrix} 1 & 2 \\ -3/2 & 3 \\ \end{matrix} \right]\]
done
clear
B)
\[\left[ \begin{matrix} 2 & -3 \\ 4 & 6 \\ \end{matrix} \right]\]
done
clear
C)
\[\left[ \begin{matrix} -2 & 4 \\ -3 & 6 \\ \end{matrix} \right]\]
done
clear
D)
does not exists
done
clear
View Answer play_arrow
question_answer 127) The distance between the foci of an ellipse is 16 and eccentricity is 1/2. Length of the major axis of the ellipse is:
A)
8
done
clear
B)
64
done
clear
C)
16
done
clear
D)
32
done
clear
View Answer play_arrow
question_answer 128) The value of \[\left[ {{\tan }^{-1}}\left( \frac{1}{2} \right)+{{\tan }^{-1}}\left( \frac{1}{3} \right) \right]\]is:
A)
\[\frac{\pi }{2}\]
done
clear
B)
\[\pi \]
done
clear
C)
\[\pi /4\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 129) If \[\cos x+\cos y+\cos \alpha =0\]and \[\sin x+\sin y+\sin \alpha =0,\] then\[\cot \left( \frac{x+y}{2} \right)=2\]
A)
\[\sin \alpha \]
done
clear
B)
\[\cos \alpha \]
done
clear
C)
\[\cot \alpha \]
done
clear
D)
\[\sin \left( \frac{x+y}{2} \right)\]
done
clear
View Answer play_arrow
question_answer 130) If we express\[\frac{{{(cos2\theta -i\sin 2\theta )}^{4}}{{(cos4\theta +i\sin 4\theta )}^{-5}}}{{{(cos3\theta +i\sin 3\theta )}^{-2}}{{(cos3\theta -i\sin 3\theta )}^{-9}}}\]in the form of\[x+iy,\]we get:
A)
\[\cos 49\theta -i\sin 49\theta \]
done
clear
B)
\[\cos 23\theta +i\sin 23\theta \]
done
clear
C)
\[\cos 49\theta +i\sin 49\theta \]
done
clear
D)
\[\cos 21\theta +i\sin 21\theta \]
done
clear
View Answer play_arrow
question_answer 131) The conjugate of\[\frac{{{(2+i)}^{2}}}{3+i},\]in the form of \[a+ib,\]is:
A)
\[\frac{13}{2}+i\left( \frac{15}{2} \right)\]
done
clear
B)
\[\frac{13}{10}+\left( \frac{-15}{2} \right)i\]
done
clear
C)
\[\frac{13}{10}+i\left( \frac{9}{10} \right)\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 132) If \[y=(1+{{x}^{2}}){{\tan }^{-1}}x-x,\]then \[\frac{dy}{dx}=\]
A)
\[{{\tan }^{-1}}x\]
done
clear
B)
\[2x{{\tan }^{-1}}x\]
done
clear
C)
\[2x{{\tan }^{-1}}x\]
done
clear
D)
\[\frac{2x}{{{\tan }^{-1}}x}\]
done
clear
View Answer play_arrow
question_answer 133) The function\[f(x)=2{{x}^{3}}-15{{x}^{2}}+36x+4\] maximum at:
A)
\[x=2\]
done
clear
B)
\[x=4\]
done
clear
C)
\[~x=0\]
done
clear
D)
\[x=3\]
done
clear
View Answer play_arrow
question_answer 134) \[\frac{d}{dx}\left[ {{\tan }^{-1}}\left( \frac{a-x}{1+ax} \right) \right]\]is equal to:
A)
\[-\frac{1}{1+{{x}^{2}}}\]
done
clear
B)
\[\frac{1}{1+{{a}^{2}}}-\frac{1}{1+{{x}^{2}}}\]
done
clear
C)
\[\frac{1}{1+{{\left( \frac{a-x}{1+ax} \right)}^{2}}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 135) If \[1,\omega ,{{\omega }^{2}}\]are the cube roots of unity, then their product is:
A)
zero
done
clear
B)
\[\omega \]
done
clear
C)
\[-1\]
done
clear
D)
\[1\]
done
clear
View Answer play_arrow
question_answer 136) The real part of \[\frac{1}{1-\cos \theta +i\sin \theta }\]is equal to:
A)
\[\frac{1}{4}\]
done
clear
B)
\[\frac{1}{2}\]
done
clear
C)
\[\tan \theta /2\]
done
clear
D)
\[\frac{1}{1-\cos \theta }\]
done
clear
View Answer play_arrow
question_answer 137) The equation of tangent at \[(-4,-4)\] on the curve\[{{x}^{2}}=-4y\] is:
A)
\[2x+y+4=0\]
done
clear
B)
\[2x-y-12=0\]
done
clear
C)
\[2x+y-4=0\]
done
clear
D)
\[2x-y+4=0\]
done
clear
View Answer play_arrow
question_answer 138) The value of=\[\int_{0}^{\pi /2}{\log \tan x\,dx}\] is:
A)
\[\infty \]
done
clear
B)
\[\pi \]
done
clear
C)
zero
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 139) The value of\[\int_{{}}^{{}}{\frac{dx}{{{(x-5)}^{2}}}}\]is:
A)
\[\frac{1}{x-5}+c\]
done
clear
B)
\[-\frac{1}{x-5}+c\]
done
clear
C)
\[\frac{2}{(x+5)}+c\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 140) The angle between the curves \[{{y}^{2}}=x\] and \[{{x}^{2}}=y\]at \[(1,1)\]is:
A)
\[{{\tan }^{-1}}(3/4)\]
done
clear
B)
\[{{\tan }^{-1}}(4/5)\]
done
clear
C)
\[{{\tan }^{-1}}(2/3)\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 141) The length of sub tangent to the curve \[{{x}^{2}}{{y}^{2}}={{a}^{4}}\] at the point \[(-a,a)\] is:
A)
3a
done
clear
B)
2a
done
clear
C)
4a
done
clear
D)
a
done
clear
View Answer play_arrow
question_answer 142) The abscissae of the points, where the tangent to curve \[y={{x}^{3}}-3{{x}^{2}}-9x+5\]is parallel to \[x-\]axis, are:
A)
\[x=0\]and 0
done
clear
B)
\[x=1\]and \[-1\]
done
clear
C)
\[~x=-1\] and 3
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 143) The general solution of the differential equation \[\frac{dy}{dx}=\frac{{{x}^{2}}}{{{y}^{2}}}\] is:
A)
\[{{x}^{3}}-{{y}^{3}}=c\]
done
clear
B)
\[{{x}^{3}}+{{y}^{3}}=c\]
done
clear
C)
\[{{x}^{2}}+{{y}^{2}}=c\]
done
clear
D)
\[{{x}^{2}}-{{y}^{2}}=c\]
done
clear
View Answer play_arrow
question_answer 144) The modulus and amplitude of \[\frac{1+i}{1-i}\] are:
A)
\[1\]and \[\frac{\pi }{4}\]
done
clear
B)
\[\sqrt{2}\]and\[\frac{\pi }{3}\]
done
clear
C)
1 and \[\frac{\pi }{2}\]
done
clear
D)
\[-1\]and \[-\pi /2\]
done
clear
View Answer play_arrow
question_answer 145) Equation of the parabola with its vertex at \[(2,1)\] and focus is \[(-1,1)\]
A)
\[4{{x}^{2}}+{{y}^{2}}-4xy+8x+46y-71=0\]
done
clear
B)
\[3{{x}^{2}}+2{{y}^{2}}-7xy+3x++9y-15=0\]
done
clear
C)
\[2{{x}^{2}}+3{{y}^{2}}-5xy+9x+7y-14=0\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 146) The length of transverse axis of the hyperbola \[3{{x}^{2}}-4{{y}^{2}}=32\] is:
A)
\[\frac{8\sqrt{2}}{\sqrt{3}}\]
done
clear
B)
\[\frac{16\sqrt{2}}{\sqrt{3}}\]
done
clear
C)
\[\frac{64}{3}\]
done
clear
D)
\[\frac{3}{32}\]
done
clear
View Answer play_arrow
question_answer 147) If \[\cos \theta =-1/2\]and \[{{0}^{o}}<\theta <{{360}^{o}},\] then the value of \[\theta \] are:
A)
\[~{{120}^{o}}\] and \[{{300}^{o}}\]
done
clear
B)
\[~{{60}^{o}}\] and \[{{120}^{o}}\]
done
clear
C)
\[{{120}^{o}}\]and \[{{240}^{o}}\]
done
clear
D)
\[~{{60}^{o}}\] and\[~{{240}^{o}}\]
done
clear
View Answer play_arrow
question_answer 148) The value of \[\left| \begin{matrix} 41 & 42 & 43 \\ 44 & 45 & 46 \\ 47 & 48 & 49 \\ \end{matrix} \right|\]is equal to:
A)
2
done
clear
B)
4
done
clear
C)
zero
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 149) If \[a\vec{i}+6\vec{j}-\vec{k}\]and \[7\vec{i}-3\vec{j}+17\vec{k}\]are perpendicular vectors, then the value of a is equal to:
A)
5
done
clear
B)
7
done
clear
C)
4
done
clear
D)
3/2
done
clear
View Answer play_arrow
question_answer 150) If A and B are square matrices and \[{{A}^{-1}}\]and\[{{B}^{-1}}\]of the same order exist, then \[{{(AB)}^{-1}}=1\]
A)
\[A{{B}^{-1}}\]
done
clear
B)
\[{{A}^{-1}}B\]
done
clear
C)
\[{{A}^{-1}}{{B}^{-1}}\]
done
clear
D)
\[{{B}^{-1}}{{A}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 151) If \[\vec{a}\]and \[\vec{b}\]are unit vectors and \[\theta \]is the angle between then \[|\vec{a}-\vec{b}|\,=\]
A)
\[2\sin \theta \]
done
clear
B)
\[2\cos \theta \]
done
clear
C)
\[2\sin \theta /2\]
done
clear
D)
\[2\cos \theta /2\]
done
clear
View Answer play_arrow
question_answer 152) If\[{{\text{H}}_{\text{1}}}\]and \[{{\text{H}}_{2}}\]are two sub groups G, then identify the correct statement:
A)
neither \[{{H}_{1}}\cup {{H}_{2}}\]nor \[{{H}_{1}}\cap {{H}_{2}}\]is a sub group
done
clear
B)
nothing can be said about \[{{H}_{1}}\cup {{H}_{2}}\]and \[{{H}_{1}}\cap {{H}_{2}}\]
done
clear
C)
\[{{H}_{1}}\cap {{H}_{2}}\] is a sub group
done
clear
D)
\[{{H}_{1}}\cup {{H}_{2}}\]is a sub group
done
clear
View Answer play_arrow
question_answer 153) The circle \[{{x}^{2}}+{{y}^{2}}-3x-4y+2=0\]cut \[x-\]axis at:
A)
\[(2,0),(-3,0)\]
done
clear
B)
\[(3,0),(4,0)\]
done
clear
C)
\[(1,0),(-1,0)\]
done
clear
D)
\[(1,0),(2,0)\]
done
clear
View Answer play_arrow
question_answer 154) The two circles \[{{x}^{2}}+{{y}^{2}}-2x+6y+6=0\]and \[{{x}^{2}}+{{y}^{2}}-5x+6y+15=0\]
A)
intersect
done
clear
B)
are concentric
done
clear
C)
touch internally
done
clear
D)
touch externally
done
clear
View Answer play_arrow
question_answer 155) The vertex of the parabola\[{{(y-2)}^{2}}=16(x-1)\]is:
A)
\[(2,1)\]
done
clear
B)
\[(1,-2)\]
done
clear
C)
\[(-1,2)\]
done
clear
D)
\[(1,2)\]
done
clear
View Answer play_arrow
question_answer 156) The unit vector perpendicular to the vectors\[\vec{i}-\vec{j}+\vec{k}\]and \[2\vec{i}+3\vec{j}-\vec{k}\]is:
A)
\[\frac{-2i+3\vec{j}+5\vec{k}}{\sqrt{30}}\]
done
clear
B)
\[\frac{-2i+5\vec{j}+6\vec{k}}{\sqrt{38}}\]
done
clear
C)
\[\frac{-2\vec{i}+3\vec{j}+5\vec{k}}{\sqrt{38}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 157) In a group G, the elements \[a,b,c,c,d\in G\]the \[{{(abc)}^{-1}}\]is:
A)
\[{{c}^{-1}}{{b}^{-1}}{{a}^{-1}}\]
done
clear
B)
\[{{b}^{-1}}{{c}^{-1}}{{d}^{-1}}\]
done
clear
C)
\[{{a}^{-1}}{{b}^{-1}}{{c}^{-1}}\]
done
clear
D)
\[{{b}^{-1}}{{c}^{-1}}{{a}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 158) If \[{{\,}^{15}}{{C}_{3r}}={{\,}^{15}}{{C}_{r+3,}}\]then r is equal to:
A)
2
done
clear
B)
4
done
clear
C)
3
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 159) The equation\[2{{x}^{2}}+4x-k{{y}^{2}}+4x+2y-1=0\]represents a pair of lines. The value of \[k\] is:
A)
\[-5/3\]
done
clear
B)
\[+\,5/3\]
done
clear
C)
\[1/3\]
done
clear
D)
\[-1/3\]
done
clear
View Answer play_arrow
question_answer 160) The equation\[12{{x}^{2}}+7xy+a{{y}^{2}}+13x-y+3=0\]represents a pair of perpendicular lines. Then the value of a is:
A)
\[7/2\]
done
clear
B)
\[-19\]
done
clear
C)
\[-12\]
done
clear
D)
\[12\]
done
clear
View Answer play_arrow
question_answer 161) \[\sqrt{2+\sqrt{2+\sqrt{2}}}+...\infty \]is equal to:
A)
\[-1\]
done
clear
B)
\[\sqrt{2}\]
done
clear
C)
2
done
clear
D)
\[1/2\]
done
clear
View Answer play_arrow
question_answer 162) If sixth term of a H.P is\[\frac{1}{61}\] and its tenth term is\[\frac{1}{105},\] then first term of that H.P. is:
A)
1/38
done
clear
B)
1/39
done
clear
C)
1/6
done
clear
D)
1/17
done
clear
View Answer play_arrow
question_answer 163) Let \[f:R\to R\] be defined by \[f(x)=3x+4.\]Then \[{{f}^{-1}}(x)\]is:
A)
\[\frac{x+4}{3}\]
done
clear
B)
\[\frac{x}{3}-4\]
done
clear
C)
\[3x+4\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 164) Let S be a finite set containing n elements. Then the total number of binary operations on S is:
A)
\[{{n}^{n}}\]
done
clear
B)
\[{{2}^{{{n}^{2}}}}\]
done
clear
C)
\[{{n}^{{{n}^{2}}}}\]
done
clear
D)
\[{{n}^{2}}\]
done
clear
View Answer play_arrow
question_answer 165) If z lies on \[|z|=1,\]then 2/z lies on:
A)
a circle
done
clear
B)
a ellipse
done
clear
C)
a straight line
done
clear
D)
a parabola
done
clear
View Answer play_arrow
question_answer 166) If \[x,l,z\]are in A.P. and \[x,2,z\]are in G.P. the \[x,4,z\]are in:
A)
A.P.
done
clear
B)
G.P.
done
clear
C)
H.P.
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 167) The roots of the equation \[\log 2({{x}^{2}}-4x+5)=(x-2)\] are:
A)
\[4,5\]
done
clear
B)
\[2,-3\]
done
clear
C)
\[~2,3\]
done
clear
D)
\[3,5\]
done
clear
View Answer play_arrow
question_answer 168) The number of ways in which 8 distinct toys can be distributed among 5 children is:
A)
\[{{5}^{8}}\]
done
clear
B)
\[{{8}^{5}}\]
done
clear
C)
\[{{\,}^{8}}{{P}_{5}}\]
done
clear
D)
\[40\]
done
clear
View Answer play_arrow
question_answer 169) in the expansion of \[{{\left( {{x}^{3}}-\frac{1}{{{x}^{2}}} \right)}^{15}},\] the constant term is:
A)
\[{{\,}^{15}}{{C}_{9}}\]
done
clear
B)
zero
done
clear
C)
\[-{{\,}^{15}}{{C}_{9}}\]
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 170) The sum of the series \[{{\log }_{4}}2-{{\log }_{8}}2+{{\log }_{16}}2...\]is:
A)
\[{{e}^{2}}\]
done
clear
B)
\[{{\log }_{e}}2+1\]
done
clear
C)
\[{{\log }_{e}}3-2\]
done
clear
D)
\[1-{{\log }_{e}}2\]
done
clear
View Answer play_arrow
question_answer 171) A root of the equation\[\left| \begin{matrix} 0 & x+a & x-b \\ x+a & 0 & x-c \\ x+b & x+c & 0 \\ \end{matrix} \right|=0\]is:
A)
\[a\]
done
clear
B)
\[b\]
done
clear
C)
zero
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 172) If the lines \[x+2ay+a=0,\]\[x+3by+b=0\]and\[x+4cy+c=0\]are concurrent, then a, b and c are in:
A)
A.P.
done
clear
B)
G.P.
done
clear
C)
H.P.
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 173) The domain of definition of the function \[f(x)=\sqrt{{{\log }_{10}}\left( \frac{5x-{{x}^{2}}}{4} \right)}\]is:
A)
\[[1,4]\]
done
clear
B)
\[(1,4)\]
done
clear
C)
\[(0,5)\]
done
clear
D)
\[(0,5]\]
done
clear
View Answer play_arrow
question_answer 174) \[\underset{x\to 0}{\mathop{\lim }}\,\frac{(1-\cos 2x)\sin 5x}{{{x}^{2}}\sin 3x}\]is equal to?
A)
10/3
done
clear
B)
3/10
done
clear
C)
6/5
done
clear
D)
5/6
done
clear
View Answer play_arrow
question_answer 175) \[\int_{0}^{1}{\frac{{{\tan }^{-1}}x}{1+{{x}^{2}}}dx}\] is equal to:
A)
\[\pi /4\]
done
clear
B)
\[{{\pi }^{2}}/32\]
done
clear
C)
1
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 176) The equation\[{{\sin }^{2}}\theta =\frac{{{x}^{2}}+{{y}^{2}}}{2xy},\] possible if:
A)
\[~x=y\]
done
clear
B)
\[x=-y\]
done
clear
C)
\[2x=y\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 177) In a group (G, *) for some a of \[G,{{a}^{2}}=e,\]where e is the identity. Then:
A)
\[a=\sqrt{e}\]
done
clear
B)
\[a={{a}^{-1}}\]
done
clear
C)
\[a=e\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 178) In a group\[G=\{0,1,2,3,4,5\}\]under addition modulo\[6,{{(2+{{3}^{-1}}+4)}^{-1}}=\]
A)
zero
done
clear
B)
2
done
clear
C)
3
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 179) \[\int_{{}}^{{}}{\frac{\cos 2\pi }{\cos \pi }}\,dx\]is equal to:
A)
\[2\sin x+\log (\sec x-\tan x)+c\]
done
clear
B)
\[2\sin x-\log (\sec x-\tan x)+c\]
done
clear
C)
\[2\sin x+\log (\sec x+\tan x)+c\]
done
clear
D)
\[2\sin -\log (\sec x+\tan x)+c\]
done
clear
View Answer play_arrow
question_answer 180) \[\int_{{}}^{{}}{\frac{x+\sin x}{1+\cos x}}\,dx\]is equal to:
A)
\[x\tan x/2+c\]
done
clear
B)
\[\cot x/2+c\]
done
clear
C)
\[\log (1+\cos x)+c\]
done
clear
D)
\[\log (x+\sin x)+c\]
done
clear
View Answer play_arrow
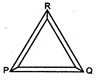 Three rods of same dimensions are arranged as shown in figure they have thermal conductivities \[{{K}_{1}},{{K}_{2}}\]and \[{{K}_{3}}.\] The points P and Q are maintained at different temperatures for the heat to flow at the same rate along PRQ and PQ:
Three rods of same dimensions are arranged as shown in figure they have thermal conductivities \[{{K}_{1}},{{K}_{2}}\]and \[{{K}_{3}}.\] The points P and Q are maintained at different temperatures for the heat to flow at the same rate along PRQ and PQ:
 The percentage of\[{{\text{N}}_{\text{2}}}\] in urea is about
The percentage of\[{{\text{N}}_{\text{2}}}\] in urea is about
