A) 2-butyne
B) 1-butene
C) propene
D) 2-butene
E) pentene
Correct Answer: D
Solution :
A compound containing a double bond can show geometrical isomerism only when identical groups are not present on the double bonded carbon atom hence, 2-butene \[(C{{H}_{3}}CH=CHC{{H}_{3}})\]shows geometrical isomerism.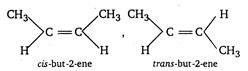
You need to login to perform this action.
You will be redirected in
3 sec
