A) ionic nature
B) acidic nature
C) covalent nature
D) electron deficient nature
Correct Answer: D
Solution :
\[{{B}_{G.S.}}1{{s}^{2}},2{{s}^{2}},2{{p}^{1}}\]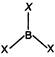 It means B has three electrons in valence shell and shares three electrons with halogens that makes total of six electrons. Hence, they are electron deficient compounds.
It means B has three electrons in valence shell and shares three electrons with halogens that makes total of six electrons. Hence, they are electron deficient compounds.
You need to login to perform this action.
You will be redirected in
3 sec
