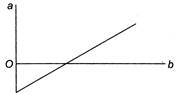
A) \[a=\]volume,\[b={}^\circ C\]temperature
B) \[a=\]volume,\[K=\]temperature
C) \[a={}^\circ C\]temperature,\[b=\]volume
D) \[a=K\]temperature,\[b=\]volume
Correct Answer: C
Solution :
For the expansion at constant pressure, Charles' law will be applied \[{{V}_{t}}={{V}_{0}}\left( 1+\frac{t}{273} \right)\] \[273{{V}_{t}}=273{{V}_{0}}+{{V}_{0}}t\] \[t=-273+\left( \frac{273}{{{V}_{0}}} \right){{V}_{t}}\] Comparing this equation with equation of straight line, \[y=mx+c\] \[\therefore \]\[a={}^\circ C\]temperature,\[b=\]volume.You need to login to perform this action.
You will be redirected in
3 sec
