Directions : In the following question more than one of the answers given may be correct. Select the correct answer and mark it according to the code:
Which of the following statements are correct? (1) A triplet carbene is more stable than a singlet carbene (2) The carbocation,\[{{F}_{3}}C\overset{+}{\mathop{C}}\,\] is less stable than the carbocation,\[{{F}_{3}}\overset{+}{\mathop{C}}\,\] (3) Methyl carbanion is both isostructural and isoelectronic with ammonia (4) A singlet carbene is a paramagnetic speciesA) 1, 2 and 3 are correct
B) 1 and 2 are correct
C) 2 and 4 are correct
D) 1 and 3 are correct
Correct Answer: A
Solution :
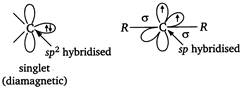 Triplet carbene is generally more stable than the singlet carbene. 2.\[{{F}_{3}}C{{C}^{+}}\]is less stable than\[{{F}_{3}}{{C}^{+}}\]. This is because of the fact that lone pairs of electrons on each of the three F -atoms in \[\overset{+}{\mathop{C}}\,{{F}_{3}}\]overlap with the empty p-orbital of the carbocation carbon. As a result, dispersal of positive charge occurs. In contrast, the strong \[-I\]effect of the three\[F-\]atoms in\[{{F}_{3}}C{{C}^{+}}\]intensifies the positive charge and thus, it is less stable than\[C{{F}_{3}}\]. 3.\[CH_{3}^{-}\Rightarrow 3bp+1\,lp=\]pyramidal geometry Number of electrons\[=6+3+1=10\] \[N{{H}_{3}}\to 3bp+1\text{ }lp=\]pyramidal geometry Number of electrons\[=7+3=10\] Thus, methyl carbanion is isostructural and isoelectronic with ammonia.
Triplet carbene is generally more stable than the singlet carbene. 2.\[{{F}_{3}}C{{C}^{+}}\]is less stable than\[{{F}_{3}}{{C}^{+}}\]. This is because of the fact that lone pairs of electrons on each of the three F -atoms in \[\overset{+}{\mathop{C}}\,{{F}_{3}}\]overlap with the empty p-orbital of the carbocation carbon. As a result, dispersal of positive charge occurs. In contrast, the strong \[-I\]effect of the three\[F-\]atoms in\[{{F}_{3}}C{{C}^{+}}\]intensifies the positive charge and thus, it is less stable than\[C{{F}_{3}}\]. 3.\[CH_{3}^{-}\Rightarrow 3bp+1\,lp=\]pyramidal geometry Number of electrons\[=6+3+1=10\] \[N{{H}_{3}}\to 3bp+1\text{ }lp=\]pyramidal geometry Number of electrons\[=7+3=10\] Thus, methyl carbanion is isostructural and isoelectronic with ammonia.
You need to login to perform this action.
You will be redirected in
3 sec
