Directions : In the following question more than one of the answers given may be correct. Select the correct answer and mark it according to the code:
Which of the following compounds will give a yellow precipitate with iodine and alkali? (1) 2-hydroxy propane (2) Acetophenone (3) Methyl acetate (4) AcetamideA) 1, 2 and 3 are correct.
B) 1 and 2 are correct.
C) 2 and 4 are correct.
D) 1 and 3 are correct.
Correct Answer: B
Solution :
A compound which contains either\[C{{H}_{3}}\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{CH}}\,\]or\[C{{H}_{3}}\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,\] group, gives yellow precipitate of iodoform with\[{{I}_{2}}\]and alkali. \[\underset{2-hydroxy\text{ }propane}{\mathop{C{{H}_{3}}-\overset{\begin{smallmatrix} OH \\ | \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{3}}}}\,\xrightarrow[-NaCl,-{{H}_{2}}O]{NaOH/{{I}_{2}}}\] \[\underset{iodoform}{\mathop{CH{{I}_{3}}}}\,+C{{H}_{3}}COONa\]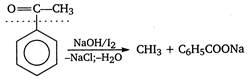 \[Methyl\text{ }acetate\xrightarrow[{}]{NaOH/{{I}_{2}}}No\text{ }reaction\] \[Acetamide\xrightarrow{NaOH/{{I}_{2}}}No\text{ }ppt\] \[\therefore \] Only 2-hydroxy propane and acetophenone give positive iodoform test.
\[Methyl\text{ }acetate\xrightarrow[{}]{NaOH/{{I}_{2}}}No\text{ }reaction\] \[Acetamide\xrightarrow{NaOH/{{I}_{2}}}No\text{ }ppt\] \[\therefore \] Only 2-hydroxy propane and acetophenone give positive iodoform test.
You need to login to perform this action.
You will be redirected in
3 sec
