question_answer 1) A body is projected at such angle that the horizontal range is three times the greatest height. The .angle of projection is
A)
\[{{42}^{o}}8\]
done
clear
B)
\[{{53}^{o}}7\]
done
clear
C)
\[{{33}^{o}}7\]
done
clear
D)
\[{{25}^{o}}8\]
done
clear
View Answer play_arrow
question_answer 2) A gas bubble formed from an explosion under water oscillates with a period T proportional to\[{{P}^{a}}{{d}^{b}}{{E}^{c}}\], where P is pressure, d is the density of water and E is the total energy of explosion. The value of a, b, c are
A)
\[a=1\], \[b=1,\,\,c=2\]
done
clear
B)
\[a=1\], \[b=2,\,\,c=1\]
done
clear
C)
\[a=\frac{5}{6}\], \[b=\frac{1}{2},c=\frac{1}{3}\]
done
clear
D)
\[a=-\frac{5}{6},b=\frac{1}{2},c=\frac{1}{3}\]
done
clear
View Answer play_arrow
question_answer 3) A particle moving with a uniform acceleration travels 24m and 64m in the first two consecutive interval of 4 s each. Its initial velocity will be
A)
5 m/s
done
clear
B)
3m/s
done
clear
C)
1 m/s
done
clear
D)
4 m/s
done
clear
View Answer play_arrow
question_answer 4) Two equal vectors have a resultant equal to either of them, then the angle between them will be
A)
\[{{120}^{o}}\]
done
clear
B)
\[{{110}^{o}}\]
done
clear
C)
\[{{60}^{o}}\]
done
clear
D)
\[{{150}^{o}}\]
done
clear
View Answer play_arrow
question_answer 5) If the radius of earth of R then the height h at which the value of g becomes one fourth, will be
A)
\[\frac{R}{8}\]
done
clear
B)
\[\frac{3R}{8}\]
done
clear
C)
\[\frac{3R}{4}\]
done
clear
D)
\[\frac{R}{2}\]
done
clear
View Answer play_arrow
question_answer 6) A body moves a distance of 10 m along a straight line under a action of 5 N force. If work done is 25 J, then angle between the force and direction of motion of the body will be
A)
\[{{75}^{o}}\]
done
clear
B)
\[{{60}^{o}}\]
done
clear
C)
\[{{45}^{o}}\]
done
clear
D)
\[{{30}^{o}}\]
done
clear
View Answer play_arrow
question_answer 7) If 150 J of heat is added to a system and the work done by the system is 110 J, then change in internal energy will be
A)
40 J
done
clear
B)
110 J
done
clear
C)
150 J
done
clear
D)
260 J
done
clear
View Answer play_arrow
question_answer 8) Sum-of the two binary numbers \[{{(100010)}_{2}}\] and \[{{(11011)}_{2}}\] is
A)
\[{{(111111)}_{2}}\]
done
clear
B)
\[{{(101111)}_{2}}\]
done
clear
C)
\[{{(111001)}_{2}}\]
done
clear
D)
\[{{(111101)}_{2}}\]
done
clear
View Answer play_arrow
question_answer 9)
What is the velocity of the bob of a simple pendulum at its mean position, if it is able to rise to vertical height of 10 cm? \[(g=9.8\,\,m/{{s}^{2}})\]
A)
2.2 m/s
done
clear
B)
1.8 m/s
done
clear
C)
1.4 m/s
done
clear
D)
0.6 m/s
done
clear
View Answer play_arrow
question_answer 10) A body cools from \[{{60}^{o}}C\] to \[{{50}^{o}}C\] in 10 min. If the room temperature is \[{{25}^{o}}C\] and assuming Newton law of cooling to hold good, the temperature of the body at the end of the next 10 min will be
A)
\[{{45}^{o}}C\]
done
clear
B)
\[{{42.85}^{o}}C\]
done
clear
C)
\[{{40}^{o}}C\]
done
clear
D)
\[{{38.5}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 11) At \[{{27}^{o}}C\] a gas suddenly compressed such that its pressure becomes \[\frac{1}{8}th\] of original pressure. The temperature of the gas will be \[(\gamma =5/3)\]
A)
\[-{{142}^{o}}C\]
done
clear
B)
300 K
done
clear
C)
\[{{327}^{o}}C\]
done
clear
D)
420 K
done
clear
View Answer play_arrow
question_answer 12) An ideal refrigerator has a freezer at a temperature of \[-{{13}^{o}}C\]. The coefficient o performance of the engine is 5. The temperature of the air (to which heat is rejected) will be
A)
\[{{325}^{o}}C\]
done
clear
B)
325 K
done
clear
C)
\[{{39}^{o}}C\]
done
clear
D)
\[{{320}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 13) In a capacitor of capacitance 20\[\mu F\] the distance between the plates is 2 mm. If a dielectric slab of width 1 mm and dielectric constant 2 is inserted between the plates, then the new capacitance will be
A)
22\[\mu F\]
done
clear
B)
26.6 \[\mu F\]
done
clear
C)
52.2 \[\mu F\]
done
clear
D)
13 \[\mu F\]
done
clear
View Answer play_arrow
question_answer 14) An automobile spring extends 0.2 m for 5000 N load. The ratio of potential energy stored in this spring when it has been compressed by 0.2 m to the potential energy stored in a 10 \[\mu F\]capacitor at a potential difference of 10000 V will be
A)
1/4
done
clear
B)
1
done
clear
C)
1/2
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 15) A solid metallic sphere has a charge + 3Q. Concentric with this sphere is a conducting spherical shell having charge - Q. The radius of the sphere is a and that of the spherical shell is\[b(b>a)\]. What is the electric field at a distance \[R(a<R<b)\] from the centre?
A)
\[\frac{4Q}{2\pi \,{{\varepsilon }_{0}}{{R}^{2}}}\]
done
clear
B)
\[\frac{3Q}{4\pi \,{{\varepsilon }_{0}}{{R}^{2}}}\]
done
clear
C)
\[\frac{3Q}{2\pi \,{{\varepsilon }_{0}}{{R}^{2}}}\]
done
clear
D)
\[\frac{Q}{2\pi \,{{\varepsilon }_{0}}R}\]
done
clear
View Answer play_arrow
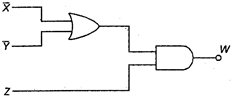
question_answer 16)
Output Y is given by :
A)
\[(\overline{X}\,.\,\,\overline{Y})\,.\,\,Z\]
done
clear
B)
\[(X\,.\,\,Y)\,.\,\,Z\]
done
clear
C)
\[(X\,+\,Y)\overline{Z}\]
done
clear
D)
\[(\overline{X}\,.\,\overline{Y}+\overline{Z}\]
done
clear
View Answer play_arrow
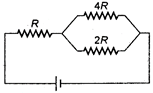
question_answer 17)
In a network as shown in the figure, the potential difference across the resistance 2R is (the cell has an emf of £ volts and has no internal resistance)
A)
2E
done
clear
B)
\[\frac{4E}{7}\]
done
clear
C)
\[\frac{E}{7}\]
done
clear
D)
E
done
clear
View Answer play_arrow
question_answer 18) The resistance of a galvanometer coil is R, then the shunt resistance required to convert it into a ammeter of range 4 times, will be
A)
4R
done
clear
B)
\[\frac{R}{3}\]
done
clear
C)
\[\frac{R}{4}\]
done
clear
D)
\[\frac{R}{5}\]
done
clear
View Answer play_arrow
question_answer 19) The instrument used by doctors for endoscopy works on the principle of
A)
total internal reflection
done
clear
B)
reflection
done
clear
C)
refraction
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 20) A meter stick is held vertically with one end on the floor and is then allowed to fall. Assuming that the end on the floor the stick does not slip, the velocity of the other end when it hits the floor, will be
A)
10.8 m/s
done
clear
B)
5.4 m/s
done
clear
C)
2.5 m/s
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 21) If the coefficient of static friction between the tyres and road is 0.5, what is the shortest distance in which an automobile can be stopped when travelling at 72 km/h?
A)
50 m
done
clear
B)
60 m
done
clear
C)
40.8 m
done
clear
D)
80.16 m
done
clear
View Answer play_arrow
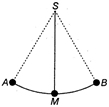
question_answer 22) A bullet fired at an angle of \[{{30}^{o}}\] with the horizontal hits the ground 3 km away. By adjusting its angle of projection, can one hope to hit a target 5 km, away. Assume the muzzle speed to be same and the air resistance is negligible
A)
possible to hit a target 5 km away
done
clear
B)
not possible to hit a target 5 km away
done
clear
C)
prediction is not possible
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 23) Two springs of spring constant 1500 N/m and 3000 N/m respectively are stretched with the same force. They will have potential energy in ratio
A)
1 : 2
done
clear
B)
2 : 1
done
clear
C)
1 : 4
done
clear
D)
4 : 1
done
clear
View Answer play_arrow
question_answer 24) The elastic energy stored in a wire of Youngs modulus Y is
A)
\[\frac{1}{2}Y\times \] stress x strain \[\times \] volume
done
clear
B)
\[\frac{{{(stress)}^{2}}\times volume}{2Y}\]
done
clear
C)
stress \[\times \]strain \[\times \] volume
done
clear
D)
\[Y\times \frac{{{(stress)}^{2}}}{volume}\]
done
clear
View Answer play_arrow
question_answer 25) A soap bubble A of radius 0.03 and another bubble B of radius 0.04 m are brought together so that the combined bubble has a common interface of radius r, then the value of r is
A)
0.24m
done
clear
B)
0.48m
done
clear
C)
0.12m
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 26) An air bubble of radius \[{{10}^{-2}}\] m is rising up at a steady rate of \[2\times {{10}^{-3}}\]m/s through a liquid of ,density \[1.5\times {{10}^{3}}kg/{{m}^{3}}\], the coefficient of viscosity neglecting the density of air, will be \[(g=10\,\,m/{{s}^{2}})\]
A)
23.2 units
done
clear
B)
83.5 units
done
clear
C)
334 units
done
clear
D)
167 units
done
clear
View Answer play_arrow
question_answer 27) A Carnot reversible engine converts 1/6 of heat input into work. When the temperature of the sink is reduced by 62 K, the efficiency of Carnots cycle becomes 1/3. The temperature of the source and sink will be
A)
372 K, 310 K
done
clear
B)
181 K, 150 K
done
clear
C)
472 K, 410 K
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 28) The ratio of the coefficient of thermal conductivity of two different materials is 5 : 3. If the thermal resistance of the rods of same thickness of these materials is same, then the ratio of the length of these rods will be
A)
3 : 5
done
clear
B)
5 : 3
done
clear
C)
3 : 4
done
clear
D)
3 : 2
done
clear
View Answer play_arrow
question_answer 29) Compressional wave pulses are sent to the bottom of sea from a ship and the echo is heard after 2 s. If bulk modulus of elasticity of water is \[2\times {{10}^{9}}N/{{m}^{2}}\] and mean temperature is \[{{4}^{o}}C\], the depth of the sea will be
A)
1014 m
done
clear
B)
1414 m
done
clear
C)
2828 m
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 30) Sound waves of \[f=600\] Hz fall normally on a perfectly reflecting wall. The shortest distance from the wall at which all particles will have maximum amplitude of vibration will be (speed of sound = 300 m/s)
A)
\[\frac{7}{8}\]m
done
clear
B)
\[\frac{3}{8}\]m
done
clear
C)
\[\frac{1}{8}\]m
done
clear
D)
\[\frac{1}{4}\]m
done
clear
View Answer play_arrow
question_answer 31) A pipe closed at one end produces a fundamental note of 412 Hz. It is cut into two pieces of equal length the fundamental notes produced by the two pieces are
A)
824 Hz, 1648 Hz
done
clear
B)
412 Hz, 824 Hz
done
clear
C)
206 Hz, 412 Hz
done
clear
D)
216 Hz, 824 Hz
done
clear
View Answer play_arrow
question_answer 32) The refractive index of water and glycerine are 1.33 and 1.47 respectively. What is the critical angle for a light ray going from the later to the former?
A)
\[{{60}^{o}}48\]
done
clear
B)
\[{{64}^{o}}48\]
done
clear
C)
\[{{74}^{o}}48\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 33) Lenses of power 3 D and -5D are combined to form a compound lens. An object is placed at a distance or 50 cm from this lens. Its image will be formed at a distance from the lens, will be
A)
25 cm
done
clear
B)
20 cm
done
clear
C)
30 cm
done
clear
D)
40 cm
done
clear
View Answer play_arrow
question_answer 34) If fringes width \[\lambda =5.89\times {{10}^{-5}}cm\] is 0.431 mm and shift of white central fringe on introducing a mica sheet in one path is 1.89 mm. Thickness of the mica sheet will be \[(\mu =1.59)\]
A)
\[4.38\times {{10}^{-6}}m\]
done
clear
B)
\[5.38\times {{10}^{-6}}m\]
done
clear
C)
\[6.38\times {{10}^{-6}}m\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 35) A body is orbiting around earth at a mean radius which is two times as greater as the parking orbit of a satellite, the period of body is
A)
4 days
done
clear
B)
16 days
done
clear
C)
\[2\sqrt{2}\]days
done
clear
D)
64 days
done
clear
View Answer play_arrow
question_answer 36) A radioactive substance has half-life of 60 min. During 3 h, the fraction of the substance that has to be decayed, will be
A)
87.5%
done
clear
B)
52.5%
done
clear
C)
25.5%
done
clear
D)
8.5%
done
clear
View Answer play_arrow
question_answer 37) Voltage in the secondary coil of a transformer does not depend upon
A)
frequency of the source
done
clear
B)
voltage in the primary coil
done
clear
C)
ratio of number of turns in the two coils
done
clear
D)
both [b] and [c]
done
clear
View Answer play_arrow
question_answer 38) When n-p-n transistor is used as an amplifier
A)
electrons move from emitter to base
done
clear
B)
electrons move from base to emitter
done
clear
C)
electrons move from collector to base
done
clear
D)
holes move from base to emitter
done
clear
View Answer play_arrow
question_answer 39) \[_{7}{{N}^{14}}\] is bombarded with \[_{2}H{{e}^{4}}\]. The resulting nucleus is \[_{8}{{O}^{17}}\] with the emission of
A)
neutrino
done
clear
B)
antineutrino
done
clear
C)
proton
done
clear
D)
neutron
done
clear
View Answer play_arrow
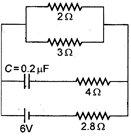
question_answer 40)
In the given figure the steady state current in the circuit i&
A)
zero
done
clear
B)
0.6 A
done
clear
C)
0.9 A
done
clear
D)
1.5 A
done
clear
View Answer play_arrow
question_answer 41) The time of vibration of a dip needle vibration in the vertical plane in the magnetic meridian is 3 s. When the same magnetic needle is made to vibrate in the horizontal plane, the time of vibration is \[3\sqrt{2}\]s. Then angle of dip will be
A)
\[{{90}^{o}}\]
done
clear
B)
\[{{60}^{o}}\]
done
clear
C)
\[{{45}^{o}}\]
done
clear
D)
\[{{30}^{o}}\]
done
clear
View Answer play_arrow
question_answer 42) The inductance of the oscillatory circuit of a radio station is 10 mH and its capacitance is 0.25\[\mu F\]. Taking the effect of resistance negligible, wavelength of the broadcasted waves will be (velocity of light\[3.0\times {{10}^{8}}\,m/s,\,\pi =3.14\])
A)
\[9.42\times {{10}^{4}}\,m\]
done
clear
B)
\[18.8\times {{10}^{4}}\,m\]
done
clear
C)
\[4.5\times {{10}^{4}}\,m\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 43) The \[{{K}_{\alpha }}\] line of singly ionised calcium has a wavelength of 393.3 nm as measured on earth. In the spectrum of one of the observed galaxies, the spectral line is located at 401.8 nm. The speed with which this galaxy is moving away from us, will be
A)
7400 m/s
done
clear
B)
\[32.4\times {{10}^{2}}\]m/s
done
clear
C)
6480 km/s
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 44) In a common-base circuit of a transistor, current amplification factor is 0.95. The base current when emitter current is 2 mA, will be
A)
0.2 mA
done
clear
B)
0.1 mA
done
clear
C)
0.002 mA
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 45) Cathode rays of velocity \[{{10}^{6}}\] m/s describe an approximate circular path of radius 1 m in an electric field 300 V/cm. If the velocity of the cathode rays are doubled. The value of electric field so that the rays describe the same circular path, will be
A)
2400 V/cm
done
clear
B)
600 V/cm
done
clear
C)
1200 V/cm
done
clear
D)
12000 V/cm
done
clear
View Answer play_arrow
question_answer 46) Light of wavelength 5000 A falling on a sensitive surface. If the surface has received \[{{10}^{-7}}J\] of energy, then the number of photons falling on the surface will be
A)
\[5\times {{10}^{11}}\]
done
clear
B)
\[2.5\times {{10}^{11}}\]
done
clear
C)
\[3\times {{10}^{11}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 47) An X-ray machine is opearated at 40 kV. The short wavelength limit of continuous X-rays will be (\[h=6.63\times {{10}^{-34}}Js,\,c=3\times {{10}^{8}}m/s\]\[,\,e=1.6\times {{10}^{-19}}C\])
A)
0.31 \[\overset{o}{\mathop{A}}\,\]
done
clear
B)
0.62 \[\overset{o}{\mathop{A}}\,\]
done
clear
C)
0.155 \[\overset{o}{\mathop{A}}\,\]
done
clear
D)
0.62 \[\overset{o}{\mathop{A}}\,\]
done
clear
View Answer play_arrow
question_answer 48) The wavelength of the first spectral line of sodium is 5896\[\overset{o}{\mathop{A}}\,\]. The first excitation potential of sodium atom will be \[(h=6.63\times {{10}^{-34}}Js)\]
A)
4.2V
done
clear
B)
3.5V
done
clear
C)
2.1V
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 49) If 200 MeV energy is released in the fission of a single nucleus of\[_{92}{{U}^{235}}\]. How many fissions must occur per second to produce a power of 1kW?
A)
\[3.125\times {{10}^{13}}\]
done
clear
B)
\[6.25\times {{10}^{13}}\]
done
clear
C)
\[1.525\times {{10}^{13}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 50) The energy supplied to calculate by state electricity board during an average November day was 40 mkh. If this energy could be obtained by the conversion of matter, how much mass will be annihilated?
A)
3.2 g
done
clear
B)
6.4 g
done
clear
C)
1.6 g
done
clear
D)
2.5 g
done
clear
View Answer play_arrow
question_answer 51) Which one of the following represents noble gas configuration?
A)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{10}},4{{s}^{2}}4{{p}^{6}}4{{d}^{10}}\], \[5{{s}^{2}},5{{p}^{6}}5{{d}^{6}},6{{s}^{2}}\]
done
clear
B)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{5}}\], \[5{{s}^{2}},5{{p}^{6}}5{{d}^{1}},6{{s}^{2}}\]
done
clear
C)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{10}},4{{s}^{2}}4{{p}^{6}}4{{d}^{10}}\], \[5{{s}^{2}}5{{p}^{6}}\]
done
clear
D)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{10}},4{{s}^{2}}4{{p}^{6}}4{{f}^{14}}\], \[5{{s}^{2}}5{{p}^{6}}5{{d}^{1}}\]
done
clear
View Answer play_arrow
question_answer 52) The number of unpaired electrons in \[Ni{{(CO)}_{4}}\]is
A)
0
done
clear
B)
1
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 53) Nitrobenzene on treatment with zinc dust and aqueous ammonium chloride gives
A)
\[{{C}_{6}}{{H}_{5}}-N=N-{{C}_{6}}{{H}_{5}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}N{{H}_{2}}\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}NO\]
done
clear
D)
\[{{C}_{6}}{{H}_{6}}NHOH\]
done
clear
View Answer play_arrow
question_answer 54) Which one of the following is a correct statement?
A)
All metal nitrates are insoluble in water
done
clear
B)
Solubility depends on temperature
done
clear
C)
All metal nitrates are soluble in water
done
clear
D)
All metal nitrates are soluble in alcohol
done
clear
View Answer play_arrow
question_answer 55) Methyl-\[\alpha \]-D-glucoside and methyl-\[\beta \]-D glucoside are
A)
epimers
done
clear
B)
anomers
done
clear
C)
enantiomers
done
clear
D)
conformational diastereomers
done
clear
View Answer play_arrow
question_answer 56) Which one of the following shows maximum value of paramagnetic behavior?
A)
\[{{[Sc{{(CN)}_{6}}]}^{3-}}\]
done
clear
B)
\[{{[Co{{(CN)}_{6}}]}^{3-}}\]
done
clear
C)
\[{{[Fe{{(CN)}_{6}}]}^{4-}}\]
done
clear
D)
\[{{[Cr{{(CN)}_{6}}]}^{3-}}\]
done
clear
View Answer play_arrow
question_answer 57) Carbolic acid is
A)
\[HCOOH\]
done
clear
B)
\[C{{H}_{3}}COOH\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}COOH\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}OH\]
done
clear
View Answer play_arrow
question_answer 58) The solubility product of \[BaC{{l}_{2}}\] is \[4\times {{10}^{-9}}\]. Its solubility in mol/L is
A)
\[4\times {{10}^{-3}}\]
done
clear
B)
\[4\times {{10}^{-9}}\]
done
clear
C)
\[1\times {{10}^{-3}}\]
done
clear
D)
\[1\times {{10}^{-9}}\]
done
clear
View Answer play_arrow
question_answer 59) Which one can differentiate between \[{{C}_{2}}{{H}_{5}}OH\] and \[C{{H}_{3}}OH\]?
A)
\[{{H}_{2}}O\]
done
clear
B)
\[N{{a}_{2}}C{{O}_{3}}+{{I}_{2}}\]
done
clear
C)
\[N{{H}_{3}}\]
done
clear
D)
\[HCl\]
done
clear
View Answer play_arrow
question_answer 60) Zinc and cold dil. \[HN{{O}_{3}}\] reacts to produce
A)
NO
done
clear
B)
\[N{{O}_{2}}\]
done
clear
C)
\[N{{H}_{4}}N{{O}_{3}}\]
done
clear
D)
\[ZnN{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 61) For the reaction, \[{{H}_{2}}+{{I}_{2}}2HI\], the equilibrium concentration of \[{{H}_{2}},\,{{I}_{2}}\] and HI are 8.0, 3.0 and 28.0 mol/L respectively. The equilibrium constant is
A)
28.34
done
clear
B)
32.66
done
clear
C)
34.78
done
clear
D)
38.88
done
clear
View Answer play_arrow
question_answer 62) Tincture of iodine is
A)
aqueous solution of \[{{I}_{2}}\]
done
clear
B)
solution of \[{{I}_{2}}\] in aqueous KI
done
clear
C)
alcoholic solution of \[{{I}_{2}}\]
done
clear
D)
aqueous solution of KI
done
clear
View Answer play_arrow
question_answer 63) The chemical formula of plaster of Paris is
A)
\[CaS{{O}_{4}}.\frac{1}{2}{{H}_{2}}O\]
done
clear
B)
\[CaS{{O}_{4}}.\,{{H}_{2}}O\]
done
clear
C)
\[CaS{{O}_{4}}.\,2{{H}_{2}}O\]
done
clear
D)
\[CaS{{O}_{4}}.\,3{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 64) Which one of the following has square planar structure?
A)
\[{{[Ni{{(CN)}_{4}}]}^{2-}}\]
done
clear
B)
\[[Ni{{(CO)}_{4}}]\]
done
clear
C)
\[{{[Ni{{(Cl)}_{4}}]}^{2-}}\]
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 65) The oxidation number of chromium in potassium dichromate is
A)
+2
done
clear
B)
+4
done
clear
C)
+6
done
clear
D)
+8
done
clear
View Answer play_arrow
question_answer 66) The electronic configuration of most electronegative elements is
A)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{5}}\]
done
clear
B)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{4}},3{{s}^{1}}\]
done
clear
C)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{1}}3{{p}^{1}}\]
done
clear
D)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{5}}\]
done
clear
View Answer play_arrow
question_answer 67) The molecular formula \[{{C}_{3}}{{H}_{9}}N\] cannot represent
A)
Famine
done
clear
B)
\[{{2}^{o}}\]amine
done
clear
C)
\[{{3}^{o}}\]amine
done
clear
D)
quaternary salt
done
clear
View Answer play_arrow
question_answer 68) The bond length between \[C-C\] bond in \[s{{p}^{2}}\] hybridised molecule is
A)
\[1.2\overset{o}{\mathop{A}}\,\]
done
clear
B)
\[1.39\overset{o}{\mathop{A}}\,\]
done
clear
C)
\[1.33\overset{o}{\mathop{A}}\,\]
done
clear
D)
\[1.54\overset{o}{\mathop{A}}\,\]
done
clear
View Answer play_arrow
question_answer 69) The energy ratio of a photon of wavelength \[3000\overset{o}{\mathop{A}}\,\] and \[6000\overset{o}{\mathop{A}}\,\] is
A)
1 : 1
done
clear
B)
2 : 1
done
clear
C)
1 : 2
done
clear
D)
1 : 4
done
clear
View Answer play_arrow
question_answer 70) Gas equation \[PV=nRT\] is obeyed by ideal gas in
A)
adiabatic process
done
clear
B)
isothermal process
done
clear
C)
both [a] and [b]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 71) Clemmensens reduction of ketones is carried out in
A)
\[LiAl{{H}_{4}}\] in \[{{H}_{2}}O\]
done
clear
B)
glycol and \[KOH\]
done
clear
C)
Zn-Hg and \[HCl\]
done
clear
D)
\[{{H}_{2}}\] and Pd catalyst
done
clear
View Answer play_arrow
question_answer 72) The planar structure of \[B{{F}_{3}}\] can be explained by the fact that \[B{{F}_{3}}\] is
A)
sp-hybridised
done
clear
B)
\[s{{p}^{2}}\]-hybridised
done
clear
C)
\[s{{p}^{3}}\]-hybridised
done
clear
D)
\[s{{p}^{3}}\] hybridized
done
clear
View Answer play_arrow
question_answer 73) Reduction of aniline with acetyl chloride in presence of \[NaOH\] produce
A)
aniline hydrochloride
done
clear
B)
acetanilide
done
clear
C)
p-chloroaniline
done
clear
D)
a red dye
done
clear
View Answer play_arrow
question_answer 74) Which of the following compounds has the highest boiling point?
A)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}Cl\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}Cl\]
done
clear
C)
\[C{{H}_{3}}CH(C{{H}_{3}})C{{H}_{2}}Cl\]
done
clear
D)
\[{{(C{{H}_{3}})}_{3}}CCl\]
done
clear
View Answer play_arrow
question_answer 75) An unknown compound D first oxidised to aldehyde and then acetic acid by a dilute solution of \[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\] and \[{{H}_{2}}S{{O}_{4}}\]. The compound D is
A)
\[C{{H}_{3}}OH\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}COOH\]
done
clear
D)
\[C{{H}_{2}}C{{H}_{2}}CHO\]
done
clear
View Answer play_arrow
question_answer 76) Which of the following is not possible?
A)
\[n=2,\,l=1,\,\,m=0\]
done
clear
B)
\[n=2,\,l=0,\,\,m=-1\]
done
clear
C)
\[n=3,\,l=0,\,\,m=0\]
done
clear
D)
\[n=3,\,l=1,\,\,m=-1\]
done
clear
View Answer play_arrow
question_answer 77) A gas expands isothermally against a constant external pressure of 1 atm from a volume of \[10\,\,d{{m}^{3}}\]to a volume of \[20\,\,d{{m}^{3}}\]. It absorbs 300 J of thermal energy from its surroundings. The \[\Delta U\] is
A)
-312J
done
clear
B)
+123J
done
clear
C)
-213J
done
clear
D)
+ 231 J
done
clear
View Answer play_arrow
question_answer 78) Phenol is more acidic than alcohol because
A)
phenol is more soluble in polar solvents
done
clear
B)
alcohol does not lose hydrogen atom
done
clear
C)
phenoxide ion is stabilised by resonance
done
clear
D)
phenoxide ion do not exhibit resonance
done
clear
View Answer play_arrow
question_answer 79) The element having highest electron affinity is
A)
bromine
done
clear
B)
iodine
done
clear
C)
fluorine
done
clear
D)
chlorine
done
clear
View Answer play_arrow
question_answer 80) Bakelite is prepared by the reaction between
A)
phenol and formaldehyde
done
clear
B)
urea and formaldehyde
done
clear
C)
ethylene and glycol
done
clear
D)
tetramethylene and glycol
done
clear
View Answer play_arrow
question_answer 81) Which one of the following is a conjugated protein?
A)
Phosphoprotein
done
clear
B)
Glycoprotein
done
clear
C)
Chromoprotein
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 82) Iodine test is shown by
A)
glucose
done
clear
B)
starch
done
clear
C)
glycogen
done
clear
D)
polypeptide
done
clear
View Answer play_arrow
question_answer 83) A fruity smell is obtained by the reaction of ethanol with
A)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
B)
\[PC{{l}_{5}}\]
done
clear
C)
\[C{{H}_{3}}COOH\]
done
clear
D)
\[C{{H}_{3}}CHO\]
done
clear
View Answer play_arrow
question_answer 84) Cyanide process is used for extraction of
A)
Ag
done
clear
B)
Ni
done
clear
C)
Pt
done
clear
D)
Zn
done
clear
View Answer play_arrow
question_answer 85) An acid solution of 0.005 M has a pH of 5. The degree of ionisation of acid is
A)
\[0.1\times {{10}^{-2}}\]
done
clear
B)
\[0.2\times {{10}^{-2}}\]
done
clear
C)
\[0.5\times {{10}^{-4}}\]
done
clear
D)
\[0.6\times {{10}^{-6}}\]
done
clear
View Answer play_arrow
question_answer 86) Which metal gives hydrogen gas on heating with hot concentrated alkali?
A)
Ag
done
clear
B)
Ni
done
clear
C)
Zn
done
clear
D)
Cu
done
clear
View Answer play_arrow
question_answer 87) The conversion of ethyl chloride into diethyl ether takes place by
A)
Williamsons synthesis
done
clear
B)
Perkins reaction
done
clear
C)
Wurtz reaction
done
clear
D)
Grignard reaction
done
clear
View Answer play_arrow
question_answer 88) \[CHC{{l}_{3}}+{{C}_{6}}{{H}_{5}}N{{H}_{2}}+3NaOH\xrightarrow{{}}A\]\[+3B+3C\] In the above reaction, the product A is
A)
chlorobenzene
done
clear
B)
phenyl isocyanide
done
clear
C)
phenyl cyanide
done
clear
D)
phenyl chloride
done
clear
View Answer play_arrow
question_answer 89) A gas is found to have a formula \[{{[CO]}_{x}}\]. Its vapour density is 70, the \[x\] is
A)
3.0
done
clear
B)
3.5
done
clear
C)
5.0
done
clear
D)
6.5
done
clear
View Answer play_arrow
question_answer 90) Which of the following is used as purgative?
A)
\[HgS\]
done
clear
B)
\[H{{g}_{2}}C{{l}_{2}}\]
done
clear
C)
\[HgC{{l}_{2}}\]
done
clear
D)
\[ZnS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 91) Least stable oxide of chlorine is
A)
\[C{{l}_{2}}O\]
done
clear
B)
\[Cl{{O}_{2}}\]
done
clear
C)
\[C{{l}_{2}}{{O}_{7}}\]
done
clear
D)
\[Cl{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 92) The sides of safety matches contains
A)
red phosphorus + sand powder
done
clear
B)
\[{{P}_{4}}{{S}_{3}}\]
done
clear
C)
\[C{{a}_{3}}{{(PO)}_{4}}+\] glass pieces
done
clear
D)
\[KCl{{O}_{3}},\,KN{{O}_{3}}\], sulphur + antimony
done
clear
View Answer play_arrow
question_answer 93) Chemically aspirin is known as
A)
salicylic acid
done
clear
B)
salicylaldehyde
done
clear
C)
2-acetoxybenzoic acid
done
clear
D)
phenyl salicylate
done
clear
View Answer play_arrow
question_answer 94) Gun metal is
A)
Cu + Zn
done
clear
B)
Cu + Sn + Zn
done
clear
C)
Cu + Sn
done
clear
D)
Zn + Sn
done
clear
View Answer play_arrow
question_answer 95) Which cannot be oxidised by \[{{H}_{2}}{{O}_{2}}\]?
A)
\[N{{a}_{2}}S{{O}_{3}}\]
done
clear
B)
\[PbS\]
done
clear
C)
KI
done
clear
D)
\[{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 96) The gas not absorbed by coconut charcoal is
A)
He
done
clear
B)
Ne
done
clear
C)
Ar
done
clear
D)
Kr
done
clear
View Answer play_arrow
question_answer 97) KI and \[CuS{{O}_{4}}\] solutions on mixing produce
A)
\[C{{u}_{2}}{{I}_{2}}+{{K}_{2}}S{{O}_{4}}\]
done
clear
B)
\[C{{u}_{2}}{{I}_{2}}+{{I}_{2}}+{{K}_{2}}S{{O}_{4}}\]
done
clear
C)
\[Cu{{I}_{2}}+{{K}_{2}}S{{O}_{4}}\]
done
clear
D)
\[Cu{{I}_{2}}+{{I}_{2}}+{{K}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 98) Vitamin \[{{B}_{12}}\] contains
A)
Co
done
clear
B)
Mn
done
clear
C)
Mg
done
clear
D)
Fe
done
clear
View Answer play_arrow
question_answer 99) Water glass is
A)
glass made of water
done
clear
B)
sodium silicate
done
clear
C)
calcium formate
done
clear
D)
pyrex glass.
done
clear
View Answer play_arrow
question_answer 100) Purification of alumina takes place by
A)
Bosch process
done
clear
B)
Halls process
done
clear
C)
Hoopes process
done
clear
D)
Quartation process
done
clear
View Answer play_arrow
question_answer 101) Incubation period of Plasmodium vivax is
A)
14 days
done
clear
B)
30 days
done
clear
C)
40 days
done
clear
D)
32 days
done
clear
View Answer play_arrow
question_answer 102) The canal system is characteristic feature of
A)
helminthes
done
clear
B)
coelenterates
done
clear
C)
sponges
done
clear
D)
echinoderms
done
clear
View Answer play_arrow
question_answer 103) In cockroach, excretion occurs through
A)
nephral glands
done
clear
B)
nephridia
done
clear
C)
malpighian tubules
done
clear
D)
parotid gland
done
clear
View Answer play_arrow
question_answer 104) antibodies are
A)
lipids
done
clear
B)
carbohydrates
done
clear
C)
immunoglobulins
done
clear
D)
antiviral particles
done
clear
View Answer play_arrow
question_answer 105) Which of the following is a viral disease?
A)
TB
done
clear
B)
Diphtheria
done
clear
C)
Small pox
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 106) In 28 days human ovarian cycle, the ovulation takes place typically on
A)
day 1st of cycle
done
clear
B)
day 6th of cycle
done
clear
C)
day 10th of cycle
done
clear
D)
day 14th of cycle
done
clear
View Answer play_arrow
question_answer 107) Trypsinogen is converted to active trypsin by
A)
enterokinase
done
clear
B)
secretins
done
clear
C)
enterocrinin
done
clear
D)
ptylin
done
clear
View Answer play_arrow
question_answer 108) Bone marrow is absent in
A)
reptiles
done
clear
B)
amphibians
done
clear
C)
aves
done
clear
D)
mammals
done
clear
View Answer play_arrow
question_answer 109) Fluid mosaic model of cell membrane was given by
A)
Singer and Tatum
done
clear
B)
Watson
done
clear
C)
Robertson
done
clear
D)
Singer and Nicolson
done
clear
View Answer play_arrow
question_answer 110) Which of the following is a connecting link between glycolysis and Krebs cycle?
A)
Citric acid
done
clear
B)
Pyruvic acid
done
clear
C)
Acetyl Co-A
done
clear
D)
Glucose
done
clear
View Answer play_arrow
question_answer 111) Lock and key model of enzyme action was proposed by
A)
Kuhne
done
clear
B)
Koshland
done
clear
C)
Buchner
done
clear
D)
Fischer
done
clear
View Answer play_arrow
question_answer 112) Which of the following process needs bacteriophage?
A)
Transduction
done
clear
B)
Translation
done
clear
C)
Transformation
done
clear
D)
Conjugation
done
clear
View Answer play_arrow
question_answer 113) Purkinje fibres are present in
A)
brain
done
clear
B)
heart
done
clear
C)
blood
done
clear
D)
lungs
done
clear
View Answer play_arrow
question_answer 114) Dissociation curve of haemoglobin is
A)
sigmoid
done
clear
B)
parabolic
done
clear
C)
straight line
done
clear
D)
hyperbolic
done
clear
View Answer play_arrow
question_answer 115) The function of Henles loop is
A)
passage of urine
done
clear
B)
formation of urine
done
clear
C)
conservation of water
done
clear
D)
filteration of water
done
clear
View Answer play_arrow
question_answer 116) Parathormone is responsible for
A)
controlling calcium level in blood
done
clear
B)
decreasing calcium level in blood
done
clear
C)
filteration in nephron
done
clear
D)
increasing absorption of water
done
clear
View Answer play_arrow
question_answer 117) Hinge joint is present between
A)
humerus and ulna
done
clear
B)
femur and pectoral girdle
done
clear
C)
humerus and pelvic girdle
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 118) Pancreas secretes pancreatic juice on stimulation by
A)
renin
done
clear
B)
gastrin
done
clear
C)
secretin
done
clear
D)
DDK
done
clear
View Answer play_arrow
question_answer 119) Which of the following is not a granulocyte?
A)
Basophils
done
clear
B)
Monocytes
done
clear
C)
Acidophils
done
clear
D)
Neutrophils
done
clear
View Answer play_arrow
question_answer 120) Nerve impulse initiates with the movements of
A)
\[{{K}^{+}}\]
done
clear
B)
\[N{{a}^{+}}\]
done
clear
C)
\[C{{a}^{+}}\]
done
clear
D)
\[M{{g}^{+}}\]
done
clear
View Answer play_arrow
question_answer 121) When an animal has both sexes (male and female), it is called
A)
intersex
done
clear
B)
super male
done
clear
C)
gynandromorph
done
clear
D)
super female
done
clear
View Answer play_arrow
question_answer 122) The contraction of gall bladder is due to
A)
gastrin
done
clear
B)
cholecystokinin
done
clear
C)
secretin
done
clear
D)
kinase
done
clear
View Answer play_arrow
question_answer 123) Which of the following has discoidal placenta?
A)
Rabbit
done
clear
B)
Deer
done
clear
C)
Sheep
done
clear
D)
Pig
done
clear
View Answer play_arrow
question_answer 124) DNA replication takes place in
A)
\[{{G}_{1}}\]-phase
done
clear
B)
S-phase
done
clear
C)
\[{{G}_{2}}\]-phase
done
clear
D)
Prophase
done
clear
View Answer play_arrow
question_answer 125) Which of the following is a transparent tissue?
A)
Tendon
done
clear
B)
Hyaline cartilage
done
clear
C)
Fibrous cartilage
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 126) A nucleoside is
A)
purine/pyramidine + phosphate
done
clear
B)
purine/pyramidine + sugar
done
clear
C)
pyramidine + purine + phosphate
done
clear
D)
purine + sugar + phosphate
done
clear
View Answer play_arrow
question_answer 127)
Floral formula
A)
Papilionasae
done
clear
B)
Mimosoideae
done
clear
C)
Ceasalpinoidae
done
clear
D)
Malvaceae
done
clear
View Answer play_arrow
question_answer 128) Formation of diploid embryosac from diploid vegetative structure, eg, nucellus or integument etc., without meiosis is called
A)
apospory
done
clear
B)
apomixis
done
clear
C)
diplospory
done
clear
D)
adventive polyembryony
done
clear
View Answer play_arrow
question_answer 129) Process of water exudation through hydathodes is known as
A)
guttation
done
clear
B)
transpiration
done
clear
C)
evaporation
done
clear
D)
bleeding
done
clear
View Answer play_arrow
question_answer 130) Insectivorous plants grow in
A)
nitrogen rich soil
done
clear
B)
nitrogen deficient soil
done
clear
C)
potassium deficient soil
done
clear
D)
carbohydrate rich soil
done
clear
View Answer play_arrow
question_answer 131) Fungus without mycelium is
A)
Puccinia
done
clear
B)
Rhizopus
done
clear
C)
Saccharomyces
done
clear
D)
Mucor
done
clear
View Answer play_arrow
question_answer 132) The organelles involved in photorespiration are
A)
glyoxisome, chloroplast and mitochondria
done
clear
B)
chloroplast, peroxisome and glyoxisome
done
clear
C)
mitochondria, peroxisome and glyoxisome
done
clear
D)
chloroplast, mitochondria and peroxisome
done
clear
View Answer play_arrow
question_answer 133) Floridean starch is found in
A)
Chlorophyceae
done
clear
B)
Myxophyceae
done
clear
C)
Phaeophyceae
done
clear
D)
Rhodophyceae
done
clear
View Answer play_arrow
question_answer 134) The hormone reducing transpiration rate by inducing stomatal closure is
A)
ABA
done
clear
B)
ethylene
done
clear
C)
cytokinin
done
clear
D)
auxin
done
clear
View Answer play_arrow
question_answer 135) The pigment involved in photomorphogenetic movements is
A)
cytochrome
done
clear
B)
phytochrome
done
clear
C)
chromatin
done
clear
D)
vernalin
done
clear
View Answer play_arrow
question_answer 136) First \[C{{O}_{2}}\] receptor in \[{{C}_{4}}\] plants is
A)
PGA
done
clear
B)
PEP
done
clear
C)
RuBP
done
clear
D)
OAA
done
clear
View Answer play_arrow
question_answer 137) The components of an ecosystem are
A)
, trees and weeds
done
clear
B)
plants and animals
done
clear
C)
man and plants
done
clear
D)
biotic and abiotic
done
clear
View Answer play_arrow
question_answer 138) The 10% energy transfer law of food chain was given by
A)
Lederberg
done
clear
B)
Lindmann
done
clear
C)
Weismann
done
clear
D)
Lindley
done
clear
View Answer play_arrow
question_answer 139) Reaction centre of photosystem-I in green plants is
A)
\[{{P}_{680}}~\]
done
clear
B)
\[{{P}_{690}}~\]
done
clear
C)
\[{{P}_{700}}~\]
done
clear
D)
\[{{P}_{780}}~\]
done
clear
View Answer play_arrow
question_answer 140) Double fertilization is found in
A)
bryophytes
done
clear
B)
angiosperms
done
clear
C)
gymnosperms
done
clear
D)
pteridophytes
done
clear
View Answer play_arrow
question_answer 141) A sporangium derived from a single cell is called
A)
leptosporangiate
done
clear
B)
eusporangiate
done
clear
C)
heterosporangiate
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 142) Root cap is not found in
A)
mesophytes
done
clear
B)
xerophytes
done
clear
C)
hydrophytes
done
clear
D)
halophytes
done
clear
View Answer play_arrow
question_answer 143) The high yielding hybrid crop varieties to exploit hybrid vigour, the farmers need to purchase fresh hybrid seed every year because
A)
hybrid vigour is not long standing due to inbreeding depression
done
clear
B)
they are not allowed to grow their own seed
done
clear
C)
it is always associated with increased heterozygosity
done
clear
D)
government has accepted Dunkels proposals
done
clear
View Answer play_arrow
question_answer 144) Genetic engineering is related with
A)
eugenics
done
clear
B)
euphenics
done
clear
C)
euthenics
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 145) Floating roots are the characteristic feature of
A)
Viscum
done
clear
B)
Cuscuta
done
clear
C)
Vanda
done
clear
D)
Jussiaea
done
clear
View Answer play_arrow
question_answer 146) The diameter of Z-DNA is
A)
34 \[\overset{o}{\mathop{A}}\,\]
done
clear
B)
20 \[\overset{o}{\mathop{A}}\,\]
done
clear
C)
18 \[\overset{o}{\mathop{A}}\,\]
done
clear
D)
45 \[\overset{o}{\mathop{A}}\,\]
done
clear
View Answer play_arrow
question_answer 147) The genes, which remain confined to differential region of Y chromosome, are
A)
autosomal genes
done
clear
B)
holandric genes
done
clear
C)
sex linked genes
done
clear
D)
mutant genes
done
clear
View Answer play_arrow
question_answer 148) The hormone responsible for ripening of fruits is
A)
ethylene
done
clear
B)
cytokinin
done
clear
C)
auxin
done
clear
D)
ABA
done
clear
View Answer play_arrow
question_answer 149) Select the correct statement.
A)
legumes are incapable of fixing nitrogen
done
clear
B)
legumes fix nitrogen through bacteria living in fruits root
done
clear
C)
legumes fix nitrogen only by bacteria present in nodules
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 150) The part of cotton producing pure cellulose is
A)
root hair
done
clear
B)
leaf hair
done
clear
C)
seed hair
done
clear
D)
stem hair
done
clear
View Answer play_arrow