A) 4
B) 1
C) 2
D) None of these
Correct Answer: C
Solution :
Key Idea Potash alum is a double salt. Potash alum, \[{{K}_{2}}S{{O}_{4}}\,.\,A{{l}_{x}}{{(S{{O}_{4}})}_{3}}\,.\,\,24{{H}_{2}}O\](given) ions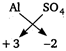 valency +3 - 2 Therefore, \[A{{l}_{2}}{{(S{{O}_{4}})}_{3}}\] is compound of \[A{{l}^{3+}}\] and \[SO_{4}^{2-}\]. On comparing, \[x=2\] Hence, formula of potash alum is = \[{{K}_{2}}S{{O}_{4}}\,.\,\,A{{l}_{2}}{{(S{{O}_{4}})}_{3}}\,.\,24{{H}_{2}}O\]
valency +3 - 2 Therefore, \[A{{l}_{2}}{{(S{{O}_{4}})}_{3}}\] is compound of \[A{{l}^{3+}}\] and \[SO_{4}^{2-}\]. On comparing, \[x=2\] Hence, formula of potash alum is = \[{{K}_{2}}S{{O}_{4}}\,.\,\,A{{l}_{2}}{{(S{{O}_{4}})}_{3}}\,.\,24{{H}_{2}}O\]
You need to login to perform this action.
You will be redirected in
3 sec
