A) \[~\text{III }<\text{ II }<\text{ IV }<\text{ I}\]
B) \[\text{II }<\text{ I }<\text{ IV }<\text{ II}\]
C) \[~\text{IV }<\text{ III }<\text{ II }<\text{ I}\]
D) \[~\text{II }<\text{ IV }<\text{ I }<\text{ III}\]
Correct Answer: D
Solution :
More electron withdrawing group favours NA reaction (nucleophilic addition reaction), whereas electron donating group decreases reactivity towards NA reaction or reactivity towards HCN. Decreasing order of NA reactivity.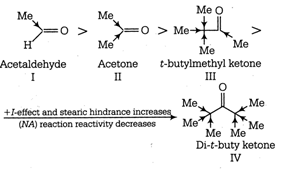 So, increasing order of reactivity towards HCN \[\text{IV III II I}\]
So, increasing order of reactivity towards HCN \[\text{IV III II I}\]
You need to login to perform this action.
You will be redirected in
3 sec
