A) \[C{{O}_{2}}\] and \[\text{Si}{{\text{O}}_{2}}\] both are gases
B) \[C{{O}_{2}}\] is gas and \[Si{{O}_{2}}\] is a liquid
C) \[C{{O}_{2}}\] is a gas but \[Si{{O}_{2}}\] is a solid
D) Carbon and Si both form oxides of similar properties
Correct Answer: C
Solution :
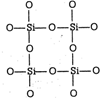
\[C{{O}_{2}}\] is a gas but \[Si{{O}_{2}}\]is a solid Carbon and silicon (group 14, IVA) have four valence electrons. We might expect carbon and silicon to form oxides with similar properties. In \[C{{O}_{2}}\] the ability of C and O-atoms to form \[\pi \]-bonds through the side-wise overlap of their 2p orbitals is strong. The result is strong \[\text{C}\text{to }O\]double bonds and a very stable triatomic molecule. \[O=\text{C}=O\] Silicon, being in third period, would have to use 3p orbitals to form double bonds with oxygen. The side-wise overlap of these orbitals with the 2p orbitals of oxygen is too limited for \[\pi \]-bond formation. From an energy stand point, a stronger bonding arrangement results if the Si-atoms form four single bonds with O-atoms (bond energy : 464kJ/mol) rather than two double bonds (bond energy : 640 kJ/mol) since each O-atom must be simultaneously bonded to two Si-atoms, the result is a network of \[-Si-O-Si-\]bonds and thus a hard (giant) solid.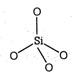
You need to login to perform this action.
You will be redirected in
3 sec
