A) \[{{\text{ }\!\![\!\!\text{ NiC}{{\text{l}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}\] is square planar and\[{{\text{ }\!\![\!\!\text{ Ni(CN}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}},\] \[\text{Ni}{{\left( \text{CO} \right)}_{\text{4}}}\] are tetrahedral
B) \[\text{Ni}{{\left( \text{CO} \right)}_{\text{4}}}\] is square planar and\[\left[ \text{Ni}{{\left( \text{CN} \right)}_{\text{4}}} \right]{{\text{2}}^{-}},\] \[{{\left[ \text{NiC}{{\text{l}}_{\text{4}}} \right]}^{\text{2-}}}\] are tetrahedral
C) \[{{\left[ \text{Ni(CN}{{\text{)}}_{\text{4}}} \right]}^{\text{2-}}}\] is square planar and\[{{\left[ \text{NiC}{{\text{l}}_{\text{4}}} \right]}^{\text{2-}}},\] \[\text{Ni}{{\left( \text{CO} \right)}_{\text{4}}}\] are tetrahedral
D) None of the above
Correct Answer: C
Solution :
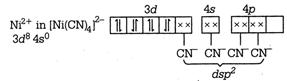
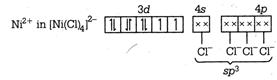
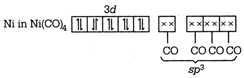 \[{{\text{ }\!\![\!\!\text{ Ni(CN}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2}}}\text{-ds}{{\text{p}}^{\text{2}}}\] \[\to \]Square planar \[{{[NiC{{l}_{4}}]}^{2-}}-s{{p}^{3}}\] \[\to \]Tetrahedral \[Ni{{(CO)}_{4}}-s{{p}^{3}}\to \]Tetrahedral
\[{{\text{ }\!\![\!\!\text{ Ni(CN}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2}}}\text{-ds}{{\text{p}}^{\text{2}}}\] \[\to \]Square planar \[{{[NiC{{l}_{4}}]}^{2-}}-s{{p}^{3}}\] \[\to \]Tetrahedral \[Ni{{(CO)}_{4}}-s{{p}^{3}}\to \]Tetrahedral
You need to login to perform this action.
You will be redirected in
3 sec
