A) \[{{P}_{4}}\] is feasible but \[{{\text{N}}_{\text{4}}}\]is not feasible
B) \[{{P}_{4}}\] is not feasible but \[{{\text{N}}_{\text{4}}}\]is feasible
C) Both \[{{\text{P}}_{4}}\] and \[{{\text{N}}_{\text{4}}}\]are feasible
D) Both \[{{P}_{4}}\] and \[{{\text{N}}_{\text{4}}}\]are not feasible
Correct Answer: A
Solution :
The process involves replacement of \[\text{2}\left( \text{X}\equiv \text{X} \right)\] units by 6 X?X bonds in the \[{{X}_{4}}\] tetrahedron. Energy is released when bonds are formed and thus species formed is stable. (i) \[2{{P}^{2}}(g)\to {{P}_{4}}(g)\]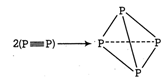 \[\Delta {{H}_{t}}=-6\times BE(P-P)+2BE(P\equiv P)\] (BE =Bonds energy ) \[=-6\times 209+2\times 490=-2\text{74 kJ mo}{{\text{l}}^{\text{-}}}\] Formation of \[{{P}_{4}}\] is exothermic thus is feasible. (ii) \[2{{N}_{2}}(g)\to {{N}_{4}}(g)\] \[\Delta {{H}_{t}}=-6BE(N-N)+2BE(N\equiv N)\] \[=-6\times 160+2\times 946=+9\text{32 kJ mo}{{\text{l}}^{\text{-1}}}\] Formation of \[{{N}_{4}}\] is endothermic, thus is not feasible.
\[\Delta {{H}_{t}}=-6\times BE(P-P)+2BE(P\equiv P)\] (BE =Bonds energy ) \[=-6\times 209+2\times 490=-2\text{74 kJ mo}{{\text{l}}^{\text{-}}}\] Formation of \[{{P}_{4}}\] is exothermic thus is feasible. (ii) \[2{{N}_{2}}(g)\to {{N}_{4}}(g)\] \[\Delta {{H}_{t}}=-6BE(N-N)+2BE(N\equiv N)\] \[=-6\times 160+2\times 946=+9\text{32 kJ mo}{{\text{l}}^{\text{-1}}}\] Formation of \[{{N}_{4}}\] is endothermic, thus is not feasible.
You need to login to perform this action.
You will be redirected in
3 sec
