A) nucleophilic attack, transfer of \[{{\text{H}}^{\text{-}}}\] and transfer of \[{{\text{H}}^{+}}\]
B) transfer of \[{{\text{H}}^{-}}\], transfer of \[{{\text{H}}^{+}}\] and nucleophilic attack
C) transfer of \[{{\text{H}}^{+}}\] , nucleophilic attack and transfer of \[{{\text{H}}^{-}}\]
D) electrophilic attack by \[\text{O}{{\text{H}}^{-}}\], transfer of \[{{\text{H}}^{-}}\] and
Correct Answer: A
Solution :
The Cannizzaro reaction is as \[\text{HCHO + HCHO}\xrightarrow{\text{KOH(con}\text{.)}}\underset{\text{methyl alcohol}}{\mathop{\text{C}{{\text{H}}_{\text{3}}}\text{OH}}}\,\text{+}\underset{\text{acetic acid}}{\mathop{\text{HCOO}{{\text{k}}^{\text{+}}}}}\,\]The mechanism of Cannizzaro reaction is as Step I Attack of nucleophile \[\text{O}{{\text{H}}^{-}}\]to the carbonyl carbon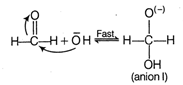 Step II The transfer of hydride ion from anion (I) to second molecule of aldehyde and finally rapid transfer of proton takes place.
Step II The transfer of hydride ion from anion (I) to second molecule of aldehyde and finally rapid transfer of proton takes place. 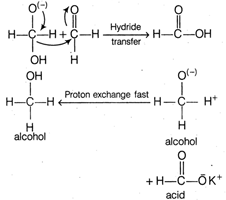
You need to login to perform this action.
You will be redirected in
3 sec
