A) \[~\text{9}0{}^\circ \]
B) \[\text{118}{}^\circ \]
C) \[\text{1}0\text{5}{}^\circ \]
D) \[\text{111}{}^\circ \]
Correct Answer: B
Solution :
The structure of \[Cl{{O}_{2}}\] is angular. The \[\text{Cl}\] -atom is \[s{{p}^{3}}\] - hybridised in the angular molecule with bond angle of \[\text{118}{}^\circ \] and \[\text{Cl }-\text{ O}\]bond length of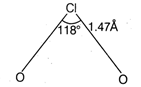
You need to login to perform this action.
You will be redirected in
3 sec
