A) Bromine is oxidised and carbonate is reduced
B) Bromine is reduced and carbonate is oxidized
C) Bromine is neither oxidised nor reduced
D) Bromine is both oxidised and reduced
Correct Answer: D
Solution :
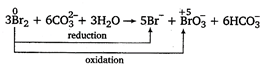 In the given reaction, bromine is oxidised as well as reduced.
In the given reaction, bromine is oxidised as well as reduced.
You need to login to perform this action.
You will be redirected in
3 sec
