A) NaCI
B) \[NaCl{{O}_{4}}\]
C) \[PC{{l}_{3}}\]
D) \[POC{{l}_{3}}\]
Correct Answer: B
Solution :
\[NaCl{{O}_{4}}\] contains both ionic and covalent bonds. Na is highly electropositive and \[ClO_{4}^{-}\]is highly electronegative, so they will form ionic bond. Perchlorate ion, \[ClO_{4}^{-}\] has a tetrahedral structure with \[s{{p}^{3}}\] hybridisation. Cl and O are bonded through covalent bonds.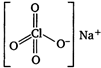
You need to login to perform this action.
You will be redirected in
3 sec
