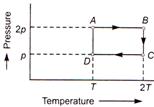
A) \[{{\text{U}}_{A}}={{U}_{D}}\]
B) \[{{\text{U}}_{B}}={{U}_{A}}\]
C) \[{{\text{U}}_{B}}={{U}_{C}}\]
D) \[{{\text{U}}_{B}}>{{U}_{C}}\]
Correct Answer: B
Solution :
Internal energy of a gas \[U=\frac{3}{2}nRT\] where R is constant. Thus for a given number of moles of the same gas \[U\propto T\] From the graphs we conclude that \[{{U}_{A}}={{U}_{D}}\] \[{{U}_{B}}={{U}_{C}}\] \[{{U}_{B}}={{U}_{A}}\] \[{{U}_{C}}={{U}_{D}}\]You need to login to perform this action.
You will be redirected in
3 sec
