A) \[PC{{l}_{3}}\]
B) \[S{{O}_{3}}\]
C) \[CO_{3}^{2-}\]
D) \[NO_{3}^{-}\]
Correct Answer: A
Solution :
In \[PC{{l}_{3}}\] molecule, phosphorus is \[s{{p}^{3}}\]-hybridized but due to presence of lone pair of electrons, it has pyramidal structure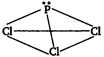
You need to login to perform this action.
You will be redirected in
3 sec
