A) 1-butene
B) 1-butyne
C) 2-butene
D) All of these
Correct Answer: C
Solution :
Key Idea Saytzeffs rule The elimination off \[\beta \]-hydrogen atom takes place from the carbon having lesser number of H-atom or in other words stable alkene is formed (more substituted alkene are more stable) The major product of dehydration of n-butyl alcohol is 2-butene. \[C{{H}_{{}}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}OH\xrightarrow{{{H}^{+}}}\]\[C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}\overset{+}{\mathop{O}}\,{{H}_{2}}\] \[\xrightarrow{-{{H}_{2}}O}C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}\overset{+}{\mathop{C}}\,{{H}_{2}}\]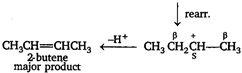 \[\underset{\begin{smallmatrix} 1-butene \\ minor\text{ }product \end{smallmatrix}}{\mathop{C{{H}_{3}}C{{H}_{2}}CH=C{{H}_{2}}}}\,\xleftarrow{-{{H}^{+}}}\] Note - \[{{2}^{o}}\] carbocation is more stable than \[{{1}^{o}}\] carbocation hence, rearrangement takes place.
\[\underset{\begin{smallmatrix} 1-butene \\ minor\text{ }product \end{smallmatrix}}{\mathop{C{{H}_{3}}C{{H}_{2}}CH=C{{H}_{2}}}}\,\xleftarrow{-{{H}^{+}}}\] Note - \[{{2}^{o}}\] carbocation is more stable than \[{{1}^{o}}\] carbocation hence, rearrangement takes place.
You need to login to perform this action.
You will be redirected in
3 sec
