A) Acid anhydride
B) Acid amide
C) Acid chloride
D) All of the above
Correct Answer: B
Solution :
Key Idea Potassium tetraiodo mercurate (II) \[{{K}_{2}}[Hg{{I}_{4}}]\] is called Nesslers reagent. It is used to test \[N{{H}_{3}}\] or \[NH_{4}^{+}\] ions. It gives brown precipitate or colouration with ammonia. Acid amide on hydrolysis will give brown precipitate with Nesslers reagent. Because on hydrolysis acid amide forms ammonium salt, which contains \[NH_{4}^{+}\] ion. \[C{{H}_{3}}CON{{H}_{2}}+HOH\xrightarrow{{}}C{{H}_{3}}COON{{H}_{4}}\] \[C{{H}_{3}}CON{{H}_{4}}C{{H}_{3}}CO{{O}^{-}}+NH_{4}^{+}\] This \[NH_{4}^{+}\] ion gives brown ppt. with Nesslers reagent. While acid anhydride and acid chloride give acetic acid on hydrolysis.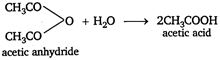 \[\underset{acetyl\text{ }chloride}{\mathop{C{{H}_{3}}COCl}}\,\,\,\,+{{H}_{2}}O\xrightarrow{{}}C{{H}_{3}}COOH+HCl\] Therefore, acid anhydride and acid chloride will not give brown ppt. with Nesslers reagent on hydrolysis.
\[\underset{acetyl\text{ }chloride}{\mathop{C{{H}_{3}}COCl}}\,\,\,\,+{{H}_{2}}O\xrightarrow{{}}C{{H}_{3}}COOH+HCl\] Therefore, acid anhydride and acid chloride will not give brown ppt. with Nesslers reagent on hydrolysis.
You need to login to perform this action.
You will be redirected in
3 sec
