A) \[{{(C{{H}_{3}})}_{3}}N\]
B) \[{{C}_{2}}{{H}_{5}}NHC{{H}_{3}}\]
C) \[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}N{{H}_{2}}\]
D) \[N{{H}_{2}}C{{H}_{2}}C{{H}_{2}}N{{H}_{2}}\]
Correct Answer: D
Solution :
Amines are polar compounds and with the exception of tertiary amines can form intermolecular hydrogen bonding. As a result amines have higher boiling points. Among the isomeric amines the primary and secondary amines have highest boiling point due to formation of H-bonds. Whereas tertiary amines have lowest boiling point because of their inability to form H-bonds. The boiling point of primary amine is greater than secondary. \[\underset{\begin{smallmatrix} p-\text{ }amine \\ b.p.:\text{ }323\text{ }K \end{smallmatrix}}{\mathop{C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}N{{H}_{2}}}}\,\,\,\underset{\begin{smallmatrix} 5-amine \\ b.p.:\text{ }307\text{ }K \end{smallmatrix}}{\mathop{C{{H}_{3}}-C{{H}_{2}}-\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{N}}\,H}}\,\,\,\,\,\underset{\begin{smallmatrix} t-amine \\ b.p.:\text{ }276\text{ }K \end{smallmatrix}}{\mathop{{{(C{{H}_{3}})}_{3}}N}}\,\]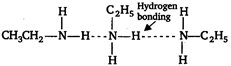 But in \[N{{H}_{2}}C{{H}_{2}}C{{H}_{2}}N{{H}_{2}}\] two amine groups are attached on both side therefore, H-bonding is maximum in this compound. Hence, its boiling point is highest.
But in \[N{{H}_{2}}C{{H}_{2}}C{{H}_{2}}N{{H}_{2}}\] two amine groups are attached on both side therefore, H-bonding is maximum in this compound. Hence, its boiling point is highest. 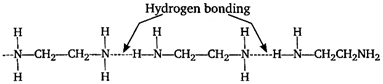
You need to login to perform this action.
You will be redirected in
3 sec
