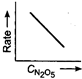
A)
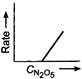
B)
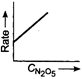
C)
D)
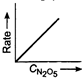
Correct Answer: D
Solution :
Key Idea If a straight line is obtained in rate versus concentration graph, then the reaction is of first order, ie, Rate \[=k\,[R]\] In the following graph, the graph is true for the first order, decomposition of \[{{N}_{2}}{{O}_{5}}\]. In this graph a straight line is obtained when it is plotted rate versus concentration, ie, Rate \[=k\,[{{N}_{2}}{{O}_{5}}]\]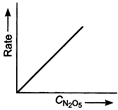 Rate of decomposition of \[\,{{N}_{2}}{{O}_{5}}\] as a function of \[\,[{{N}_{2}}{{O}_{5}}]\]. Note - In rate = \[k\,\,{{[R]}^{0,}}\] then the reaction is of zero. If rate = \[k\,\,[R]\] then the reaction is of first order. If rate = \[k\,\,{{[R]}^{2}}\], then the reaction is of second order. If rate = \[k\,\,{{[R]}^{3}}\], then the reaction is of third order.
Rate of decomposition of \[\,{{N}_{2}}{{O}_{5}}\] as a function of \[\,[{{N}_{2}}{{O}_{5}}]\]. Note - In rate = \[k\,\,{{[R]}^{0,}}\] then the reaction is of zero. If rate = \[k\,\,[R]\] then the reaction is of first order. If rate = \[k\,\,{{[R]}^{2}}\], then the reaction is of second order. If rate = \[k\,\,{{[R]}^{3}}\], then the reaction is of third order.
You need to login to perform this action.
You will be redirected in
3 sec
