A)
![]()
B)
![]()
C)
![]()
D)
![]()
Correct Answer: D
Solution :
Key Idea Conditions for geometrical isomerism The molecule must have a double bond. The two atoms or groups attached to the same carbon atom must be different.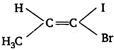 It shows geometrical isomerism because four different group/atom attached to the carbon atoms of a double bond.
It shows geometrical isomerism because four different group/atom attached to the carbon atoms of a double bond.
You need to login to perform this action.
You will be redirected in
3 sec
