A)
![]()
B)
![]()
C)
![]()
D)
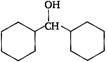
Correct Answer: B
Solution :
Key Idea Presence of electron withdrawing substituent increases the acidity while electron releasing substituent, when present, decreases the acidity. Phenyl is an electron withdrawing substituent while\[-\text{C}{{\text{H}}_{\text{3}}}\]is an electron releasing substituent. Moreover, peroxide ion is more resonance stabilized as compared to benzyl oxide ion, thus releases proton more easily. That's why is a strong acid among the given.You need to login to perform this action.
You will be redirected in
3 sec
