A) meso form
B) racemic mixture
C) d-form
D) -form
Correct Answer: B
Solution :
Chlorination of n-butane takes place by free radical mechanism as - Step I: \[C{{l}_{2}}\xrightarrow{\,}\,\overset{\centerdot }{\mathop{Cl}}\,+\overset{\centerdot }{\mathop{Cl}}\,\] Step II: \[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}+\,\overset{\centerdot }{\mathop{Cl}}\,\xrightarrow{\,}\] Step III: \[C{{H}_{3}}\overset{\centerdot }{\mathop{CH}}\,C{{H}_{2}}C{{H}_{3}}+C{{l}_{2}}\to \]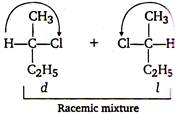
You need to login to perform this action.
You will be redirected in
3 sec
