A) sp-hybridisation
B) \[s{{p}^{2}}\]-hybridisation
C) \[s{{p}^{3}}\]-hybridisation
D) \[s{{p}^{3}}{{d}^{2}}\]-hybridisation
Correct Answer: C
Solution :
Each boron atom in diborane \[({{B}_{2}}{{H}_{6}})\] is \[s{{p}^{3}}\]. In the structure of diborane four H-atoms, two on the left and two on the right, known as terminal hydrogens, are in different environments from the other two hydrogen atoms which are known as bridging atoms. The two boron atoms and the four terminal hydrogen atoms lie in the same plane while the two boron atoms and the two bridging hydrogen atoms, one above and the other below, lie in a plane perpendicular to this plane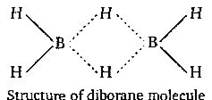
You need to login to perform this action.
You will be redirected in
3 sec
