A) there are three unpaired electrons so that its magnetic moment is 3.87 BM
B) NO transfer its electron to Fe24- so that iron as \[{{C}_{2}}{{H}_{5}}CHBr-C{{H}_{3}}\,\,and\,\,{{C}_{2}}{{H}_{5}}-C{{H}_{2}}-C{{H}_{2}}Br\]
C) the colour is because of charge transfer
D) All of the above statements are correct
Correct Answer: D
Solution :
\[\left( \text{kJ mo}{{\text{l}}^{\text{-1}}} \right)\]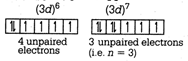 For \[{{\left[ \text{Co}{{\left( \text{N}{{\text{H}}_{\text{3}}} \right)}_{\text{4}}}\text{C}{{\text{l}}_{\text{2}}} \right]}^{+}}\] \[\text{I}\]
For \[{{\left[ \text{Co}{{\left( \text{N}{{\text{H}}_{\text{3}}} \right)}_{\text{4}}}\text{C}{{\text{l}}_{\text{2}}} \right]}^{+}}\] \[\text{I}\]
You need to login to perform this action.
You will be redirected in
3 sec
