 \[\Delta G\] \[2Zn(s)+3{{O}_{2}}(g)\to 2ZnO(g)+2S{{O}_{2}}(g)\]
\[\Delta G\] \[2Zn(s)+3{{O}_{2}}(g)\to 2ZnO(g)+2S{{O}_{2}}(g)\] 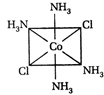
 \[\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{CH}=\text{C}{{\text{H}}_{\text{2}}}\text{+HBr}\] \[\xrightarrow{\text{ROOR (peroxide)}}\underset{Major}{\mathop{(X)}}\,+\underset{Minor}{\mathop{(Y)}}\,\]Which of the following statements is false?
\[\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{CH}=\text{C}{{\text{H}}_{\text{2}}}\text{+HBr}\] \[\xrightarrow{\text{ROOR (peroxide)}}\underset{Major}{\mathop{(X)}}\,+\underset{Minor}{\mathop{(Y)}}\,\]Which of the following statements is false?
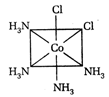
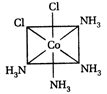
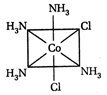
A) II and III are c/s and trans-isomers respectively
B) III and IV are trans and c/'s-isomers respectively
C) I and II are enantiomers
D) All are identical
Correct Answer: C
Solution :
 These are not enantiomers.
These are not enantiomers.
You need to login to perform this action.
You will be redirected in
3 sec
