A) \[82\]
B) \[61.5\]
C) \[41\]
D) \[20.5\]
Correct Answer: B
Solution :
The reaction can be written as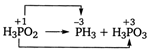 Molecular weight of \[{{H}_{3}}P{{O}_{2}}\] \[=3+31+48=82\] Equivalent weight \[=\frac{82}{4}+\frac{82}{2}=61.5\]
Molecular weight of \[{{H}_{3}}P{{O}_{2}}\] \[=3+31+48=82\] Equivalent weight \[=\frac{82}{4}+\frac{82}{2}=61.5\]
You need to login to perform this action.
You will be redirected in
3 sec
