A) tetrahedral, \[(-z-x+2y)kJ\]
B) linear, \[sp\]
C) pyramidal, \[(-x-2y-z)kJ\]
D) trigonal planar, \[(-x-2y+z)kJ\]
Correct Answer: C
Solution :
When sulphur reacts with chlorine in 1: 2 ratio, then \[BaC{{l}_{2}}\] is obtained which on hydrolysis gives sulphurous acid \[KCl\]. \[-{{2}^{o}}C\] \[BaC{{l}_{2}}\] \[-{{3}^{o}}C\] The anion of (V) is \[+{{3}^{o}}C\]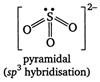
You need to login to perform this action.
You will be redirected in
3 sec
