A) (i) and (iv)
B) (i) and (ii)
C) (ii) and (iii)
D) (iii) and (iv)
Correct Answer: A
Solution :
\[{{X}_{e}}{{F}_{4}}\] In ground state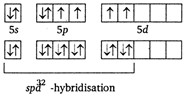
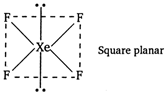 \[S{{F}_{4}}\] is distorted trigonal bipyramidal structure
\[S{{F}_{4}}\] is distorted trigonal bipyramidal structure 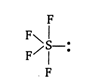 \[{{[NiC{{l}_{4}}]}^{2-}}\] \[s{{p}^{3}}\] hybridisation tetrahedral structure.
\[{{[NiC{{l}_{4}}]}^{2-}}\] \[s{{p}^{3}}\] hybridisation tetrahedral structure. 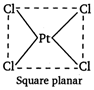 \[{{[PtC{{l}_{4}}]}^{2-}}.\] It is square planar in structure.
\[{{[PtC{{l}_{4}}]}^{2-}}.\] It is square planar in structure. 
You need to login to perform this action.
You will be redirected in
3 sec
