A)
![]()
B)
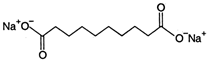
C)
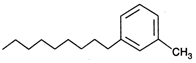
D)
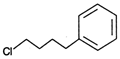
Correct Answer: C
Solution :
Benzene is non-polar, covalent organic compound. As we know that like dissolves like i.e., polar compound is soluble in polar medium and non polar soluble in non polar medium. Among the given compounds meta-methylnonyl benzene is the only non-polar compound hence it is soluble in benzene. Rest all others have polar ends.
You need to login to perform this action.
You will be redirected in
3 sec
