A) (i) and (ii) only
B) (i) and (iii) only
C) (ii) and (iii) only
D) (i), (ii) and (iii)
Correct Answer: D
Solution :
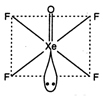 \[XeO{{F}_{4}}\]. square planar {\[s{{p}^{3}}{{a}^{2}}\] hybridization)
\[XeO{{F}_{4}}\]. square planar {\[s{{p}^{3}}{{a}^{2}}\] hybridization) 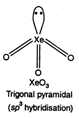
 As shown above all the compounds have one lone pair.
As shown above all the compounds have one lone pair.
You need to login to perform this action.
You will be redirected in
3 sec
