Direction for: In each of the following questions a statement of Assertion is given followed by a corresponding statement of Reason just below it. Of the statements mark the correct answer as
Assertion: In pressure-temperature (P-T) phase diagram of water, the slope of the melting curve is found to be negative. Reason: Ice contracts on melting to water.A) If both assertion and reason are true and reason is the correct explanation of assertion.
B) If both assertion and reason are true but reason is not the correct explanation of assertion.
C) If assertion is true but reason is false.
D) If both assertion and reason are false.
Correct Answer: A
Solution :
The phase diagram that is pressure temperature diagram of water is as shown. In phase diagram of water, the solid-liquid phase boundary has a negative slope. This reflects the fact that ice has a lower density than water which is an usual property i.e., ice contracts on melting to water.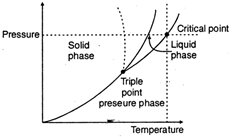
You need to login to perform this action.
You will be redirected in
3 sec
