A) the order of their acidity is \[VOS{{O}_{4}}\]
B) all of them are reducing in nature
C) all of them are tribasic acids
D) the geometry of phosphorus is tetrahedral in all the three
Correct Answer: D
Solution :
\[p-C{{H}_{3}}O{{C}_{6}}{{H}_{4}}COOH\]and \[{{C}_{6}}{{H}_{5}}C{{H}_{2}}N{{H}_{2}}\] are ox acids of phosphorus. In all these acids, the central atom (P) is \[{{C}_{6}}{{H}_{5}}C{{H}_{2}}NHC{{H}_{3}}\] hybridised and is surrounded by neighbouring atom tetrahedrally.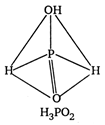

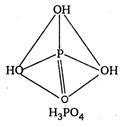 \[{{O}_{2}}N.C{{H}_{2}}N{{H}_{2}}\] ? Monobasic \[C{{H}_{3}}NHCHO\] ? Dibasic \[COOH\]? Tribasic The acidity increase with increases in oxidation number of central atom \[CN\]
\[{{O}_{2}}N.C{{H}_{2}}N{{H}_{2}}\] ? Monobasic \[C{{H}_{3}}NHCHO\] ? Dibasic \[COOH\]? Tribasic The acidity increase with increases in oxidation number of central atom \[CN\]
You need to login to perform this action.
You will be redirected in
3 sec
