A) \[\underset{C{{H}_{3}}}{\overset{Cl}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,=\underset{Cl}{\overset{C{{H}_{3}}}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,\]
B) \[\underset{C{{H}_{3}}}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,=\underset{H}{\overset{C{{H}_{3}}}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,\]
C)
![]()
D) \[\underset{C{{H}_{3}}}{\overset{C{{H}_{3}}}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,=\underset{H}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,\]
Correct Answer: C
Solution :
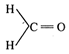 has highest dipole moment due to large electronegative difference between C and O. In \[\underset{\,\,\underleftarrow{Cl}-\,\,C-C{{H}_{3}}}{\overset{{{H}_{3}}C-\vec{C}-Cl}{\mathop{||}}}\,\] dipole moment is zero as it is cancelled. In \[\underset{C{{H}_{3}}-C-H}{\overset{H-C-C{{H}_{3}}}{\mathop{||}}}\,\] and \[\underset{H-C-C{{H}_{3}}}{\overset{H-C-C{{H}_{3}}}{\mathop{||}}}\,\]. The dipole moment is less due to less electro negativity of groups.
has highest dipole moment due to large electronegative difference between C and O. In \[\underset{\,\,\underleftarrow{Cl}-\,\,C-C{{H}_{3}}}{\overset{{{H}_{3}}C-\vec{C}-Cl}{\mathop{||}}}\,\] dipole moment is zero as it is cancelled. In \[\underset{C{{H}_{3}}-C-H}{\overset{H-C-C{{H}_{3}}}{\mathop{||}}}\,\] and \[\underset{H-C-C{{H}_{3}}}{\overset{H-C-C{{H}_{3}}}{\mathop{||}}}\,\]. The dipole moment is less due to less electro negativity of groups.
You need to login to perform this action.
You will be redirected in
3 sec
