A) \[\frac{{{({{m}_{1}}+{{m}_{2}})}^{2}}{{n}^{2}}{{h}^{2}}}{2m_{1}^{2}m_{2}^{2}{{r}^{2}}}\]
B) \[\frac{{{n}^{2}}{{h}^{2}}}{2({{m}_{1}}+{{m}_{2}}){{r}^{2}}}\]
C) \[\frac{2{{n}^{2}}{{h}^{2}}}{({{m}_{1}}+{{m}_{2}}){{r}^{2}}}\]
D) \[\frac{({{m}_{1}}+{{m}_{2}}){{n}^{2}}{{h}^{2}}}{2{{m}_{1}}{{m}_{2}}{{r}^{2}}}\]
Correct Answer: D
Solution :
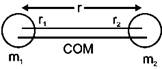 \[{{m}_{1}}{{r}_{1}}={{m}_{2}}{{r}_{2}}\] \[{{r}_{1}}+{{r}_{2}}=r\] \[\therefore \,\,{{r}_{1}}=\frac{{{m}_{2}}r}{{{m}_{1}}+{{m}_{2}}}\] \[{{r}_{2}}=\frac{{{m}_{1}}r}{{{m}_{1}}+{{m}_{2}}}\] \[\therefore \,\,\varepsilon =\frac{1}{2}I{{\omega }^{2}}\] \[=\frac{1}{2}({{m}_{1}}{{r}_{1}}^{2}+{{m}_{2}}{{r}_{2}}^{2}).\,{{\omega }^{2}}\] ........... (i) \[mvr=\frac{h}{2\pi }=I\omega \] \[\omega =\frac{nh}{2\pi I}\] \[\therefore \,\,\varepsilon =\frac{1}{2}I.\frac{{{n}^{2}}{{h}^{2}}}{4{{\pi }^{2}}{{I}^{2}}}\] \[=\frac{{{n}^{2}}{{h}^{2}}}{8{{\pi }^{2}}}\frac{1}{({{m}_{1}}r_{1}^{2}+{{m}_{2}}r_{2}^{2})}\] \[=\frac{{{n}^{2}}{{h}^{2}}}{8{{\pi }^{2}}}\frac{1}{{{m}_{1}}\frac{m_{2}^{2}r_{0}^{2}}{{{({{m}_{1}}+{{m}_{2}})}^{2}}}}+{{m}_{2}}\frac{{{m}_{1}}^{2}{{r}^{2}}}{{{({{m}_{1}}+{{m}_{2}})}^{2}}}\] \[=\frac{{{n}^{2}}{{h}^{2}}}{8{{\pi }^{2}}{{r}^{2}}}=\frac{{{({{m}_{1}}+{{m}_{2}})}^{2}}}{{{m}_{1}}{{m}_{2}}({{m}_{1}}+{{m}_{2}})}=\frac{({{m}_{1}}+{{m}_{2}}){{n}^{2}}{{h}^{2}}}{8{{\pi }^{2}}{{r}^{2}}{{m}_{1}}{{m}_{2}}}\]
\[{{m}_{1}}{{r}_{1}}={{m}_{2}}{{r}_{2}}\] \[{{r}_{1}}+{{r}_{2}}=r\] \[\therefore \,\,{{r}_{1}}=\frac{{{m}_{2}}r}{{{m}_{1}}+{{m}_{2}}}\] \[{{r}_{2}}=\frac{{{m}_{1}}r}{{{m}_{1}}+{{m}_{2}}}\] \[\therefore \,\,\varepsilon =\frac{1}{2}I{{\omega }^{2}}\] \[=\frac{1}{2}({{m}_{1}}{{r}_{1}}^{2}+{{m}_{2}}{{r}_{2}}^{2}).\,{{\omega }^{2}}\] ........... (i) \[mvr=\frac{h}{2\pi }=I\omega \] \[\omega =\frac{nh}{2\pi I}\] \[\therefore \,\,\varepsilon =\frac{1}{2}I.\frac{{{n}^{2}}{{h}^{2}}}{4{{\pi }^{2}}{{I}^{2}}}\] \[=\frac{{{n}^{2}}{{h}^{2}}}{8{{\pi }^{2}}}\frac{1}{({{m}_{1}}r_{1}^{2}+{{m}_{2}}r_{2}^{2})}\] \[=\frac{{{n}^{2}}{{h}^{2}}}{8{{\pi }^{2}}}\frac{1}{{{m}_{1}}\frac{m_{2}^{2}r_{0}^{2}}{{{({{m}_{1}}+{{m}_{2}})}^{2}}}}+{{m}_{2}}\frac{{{m}_{1}}^{2}{{r}^{2}}}{{{({{m}_{1}}+{{m}_{2}})}^{2}}}\] \[=\frac{{{n}^{2}}{{h}^{2}}}{8{{\pi }^{2}}{{r}^{2}}}=\frac{{{({{m}_{1}}+{{m}_{2}})}^{2}}}{{{m}_{1}}{{m}_{2}}({{m}_{1}}+{{m}_{2}})}=\frac{({{m}_{1}}+{{m}_{2}}){{n}^{2}}{{h}^{2}}}{8{{\pi }^{2}}{{r}^{2}}{{m}_{1}}{{m}_{2}}}\]
You need to login to perform this action.
You will be redirected in
3 sec
