A) six
B) three
C) one
D) two
Correct Answer: C
Solution :
EDTA (Ethylenediaminetetra acetic acid)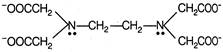 It is hexadentate that's why for octahedral complex only one EDTA is required.
It is hexadentate that's why for octahedral complex only one EDTA is required.
You need to login to perform this action.
You will be redirected in
3 sec
