A) steric hindrance
B) inductive effect
C) instability
D) insolubility
Correct Answer: A
Solution :
In\[{{S}_{N}}2\]reaction, nucleophile and alkyl halide react in one step.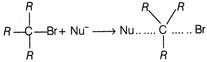 Thus, tertiary carbon is under steric hindrance thus reaction does not take place until \[(CBr)\]bond breaks \[R-\underset{\begin{smallmatrix} | \\ R \end{smallmatrix}}{\overset{\begin{smallmatrix} R \\ | \end{smallmatrix}}{\mathop{C}}}\,-Br\xrightarrow{Slow}\,R-\underset{\begin{smallmatrix} | \\ R \end{smallmatrix}}{\overset{\begin{smallmatrix} R \\ | \end{smallmatrix}}{\mathop{{{C}^{\oplus }}}}}\,+B{{r}^{-}}\] which is then\[{{S}_{N}}1\]reaction. Alternate Solution Back side attack takes place in\[{{S}_{N}}2\]reaction but it is not possible in tertiary alkyl halide because of steric hinderence. Thus, it is practically inert to substitution by\[{{S}_{N}}2\]mechanism.
Thus, tertiary carbon is under steric hindrance thus reaction does not take place until \[(CBr)\]bond breaks \[R-\underset{\begin{smallmatrix} | \\ R \end{smallmatrix}}{\overset{\begin{smallmatrix} R \\ | \end{smallmatrix}}{\mathop{C}}}\,-Br\xrightarrow{Slow}\,R-\underset{\begin{smallmatrix} | \\ R \end{smallmatrix}}{\overset{\begin{smallmatrix} R \\ | \end{smallmatrix}}{\mathop{{{C}^{\oplus }}}}}\,+B{{r}^{-}}\] which is then\[{{S}_{N}}1\]reaction. Alternate Solution Back side attack takes place in\[{{S}_{N}}2\]reaction but it is not possible in tertiary alkyl halide because of steric hinderence. Thus, it is practically inert to substitution by\[{{S}_{N}}2\]mechanism.
You need to login to perform this action.
You will be redirected in
3 sec
