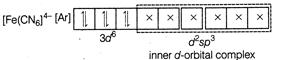
A) \[{{[Fe{{(CN)}_{6}}]}^{4-}}\]
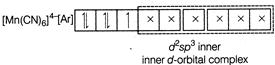
B) \[{{[Mn{{(CN)}_{6}}]}^{4-}}\]
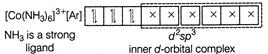
C) \[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
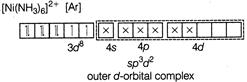
D) \[{{[Ni{{(N{{H}_{3}})}_{6}}]}^{2+}}\]
Correct Answer: D
Solution :
Complexes which use one ns, three np and two nd-orbital to form the six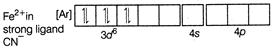
You need to login to perform this action.
You will be redirected in
3 sec
