A) \[3\to 2\]
B) \[5\to 2\]
C) \[4\to 1\]
D) \[2\to 5\]
Correct Answer: B
Solution :
Hydrogen spectrum coloured radiation means visible radiation corresponds to Balmer series \[({{n}_{1}}=2,\,{{n}_{2}}=3,4...)\]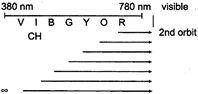 3rd line from the red end it means \[5\to 2\]
3rd line from the red end it means \[5\to 2\]
You need to login to perform this action.
You will be redirected in
3 sec
