A) elimination reaction
B) free radical substitution
C) nucleophilic substitution
D) electrophilic substitution
Correct Answer: C
Solution :
\[{{(C{{H}_{3}})}_{3}}-CBr+{{H}_{2}}O\xrightarrow{{}}{{(C{{H}_{3}})}_{3}}-C-OH\] \[+HBr\] Br is substituted by \[-O{{H}^{-}}\] (nucleophile) \[{{S}_{N}}1\] (unimolecular substitution reaction) In this reaction Br group is substituted by \[-OH\] group so it is a nucleophilic substitution reaction. The \[{{(C{{H}_{3}})}_{3}}\] C Br is \[{{3}^{o}}\] alkyl halide so it follows \[{{S}_{N}}1\] mechanism.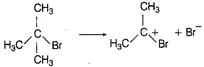
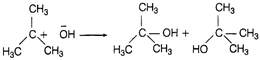 In this reaction one nucleophile (?Br) is substituted by other (?OH). So, it is a nucleophilic substitution reaction. In elimination reaction two substituents are removed from a molecule in either one or two step mechanism. In free redical substitution reaction, free radicals are the reactive intermediate while those reactions in which an electrophile displaces a functional group in a compound is known as electrophilic substitution reaction.
In this reaction one nucleophile (?Br) is substituted by other (?OH). So, it is a nucleophilic substitution reaction. In elimination reaction two substituents are removed from a molecule in either one or two step mechanism. In free redical substitution reaction, free radicals are the reactive intermediate while those reactions in which an electrophile displaces a functional group in a compound is known as electrophilic substitution reaction.
You need to login to perform this action.
You will be redirected in
3 sec
