A) \[\frac{P}{2},\frac{T}{2}\]
B) \[P,T\]
C) \[P,\frac{T}{2}\]
D) \[\frac{P}{2},T\]
Correct Answer: D
Solution :
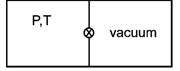 It is the free expansion So, T remain constant \[{{P}_{1}}{{V}_{1}}={{P}_{2}}{{V}_{2}}\] \[P\frac{V}{2}={{P}_{2}}(V)\] \[{{P}_{2}}=\left( \frac{P}{2} \right).\]
It is the free expansion So, T remain constant \[{{P}_{1}}{{V}_{1}}={{P}_{2}}{{V}_{2}}\] \[P\frac{V}{2}={{P}_{2}}(V)\] \[{{P}_{2}}=\left( \frac{P}{2} \right).\]
You need to login to perform this action.
You will be redirected in
3 sec
