A) \[s{{p}^{3}}\] hydridisation
B) \[s{{p}^{2}}\]hybridization
C) \[sp\]hybridisation
D) \[{{d}^{2}}s{{p}^{3}}\]hybridization
Correct Answer: C
Solution :
Carbon (At .No = 6 ) \[EC=2,\,4(1{{s}^{2}}2{{s}^{2}}2{{p}^{2}})\] in excited state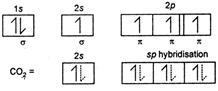 sp hybrids atom orbital which from n bond does not take part in hybridisation. Carbon has no lone pair so, according to VSEPR theory the sp hybrid orbitals stay as far away as possible. In this case, the bond angle being \[\text{18}{{\text{0}}^{\text{o}}}.\] \[s{{p}^{3}}\] hybrid molecules are tetrahedral with bond angle \[{{109}^{o}}28',\,\,s{{p}^{2}}\]hybrid orbitals are triangular planar with bond angle \[\text{12}{{\text{0}}^{\text{o}}}\text{.}\] \[\overset{s{{p}^{2}}}{\mathop{{{H}_{2}}C}}\,=\overset{s{{p}^{2}}}{\mathop{CH}}\,-\overset{sp}{\mathop{C}}\,\equiv \overset{sp}{\mathop{C}}\,-\overset{s{{p}^{3}}}{\mathop{C}}\,{{H}_{3}}\]
sp hybrids atom orbital which from n bond does not take part in hybridisation. Carbon has no lone pair so, according to VSEPR theory the sp hybrid orbitals stay as far away as possible. In this case, the bond angle being \[\text{18}{{\text{0}}^{\text{o}}}.\] \[s{{p}^{3}}\] hybrid molecules are tetrahedral with bond angle \[{{109}^{o}}28',\,\,s{{p}^{2}}\]hybrid orbitals are triangular planar with bond angle \[\text{12}{{\text{0}}^{\text{o}}}\text{.}\] \[\overset{s{{p}^{2}}}{\mathop{{{H}_{2}}C}}\,=\overset{s{{p}^{2}}}{\mathop{CH}}\,-\overset{sp}{\mathop{C}}\,\equiv \overset{sp}{\mathop{C}}\,-\overset{s{{p}^{3}}}{\mathop{C}}\,{{H}_{3}}\]
You need to login to perform this action.
You will be redirected in
3 sec
