| (a) In electrolysis of water, why is the volume of gas collected over one electrode double that of gas collected over the other electrode? |
| (b) (i) What is observed when a solution of potassium iodide is added to a solution of lead nitrate taken in a test tube? |
| (ii) What type of reaction is this? |
| (iii) Write a balanced chemical equation to represent the above reaction. |
| (a) What happens when an aqueous solution of sodium sulphate reacts with an aqueous solution of barium chloride? State the physical conditions of reactants in which reaction between them will not take place. Write the balanced chemical equation for the reaction and also mention the type of reaction. |
| (b) What changes in the colour of iron nails and copper sulphate solution do you observe after keeping the iron nails in copper sulphate solution for about half an hour? |
| (a) State reason for the following: |
| (i) Rings of cartilage are present in the trachea. |
| (ii) Plants look green in colour. |
| (b) Write other names of the following: |
| (i) Alveolar sac |
| (ii) Voice box |
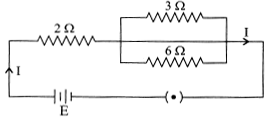
| In the given electric circuit if the current flowing through \[3\,\Omega \] resistor is 1 A, find the voltage of the battery and the current I drawn from it. |
 |
| (a) Explain any two physical properties of ionic compounds giving reason. |
| (b) List any two metals found in free state in earth?s crust. Where are they locate in activity series? |
| (c) Metals towards the top of the activity series can not be obtained from their compounds by reducing with carbon. Why? |
| (a) The blue colour of crystals of a substance changed on heating in a closed test tube but the colour was regained after sometime on cooling. Name the substance and write its chemical formula. Explain the phenomenon involved. |
| (b) Write name and chemical formula of two such compounds whose formula unit is associated with 10 and 2 water molecules respectively. |
| (a) A coil of insulated wire is connected to a galvanometer What would be seen if a bar magnet with its south pole towards one face of the coil is |
| (i) moved quickly toward it |
| (ii) moved quickly away from it |
| (iii) placed near its one face? |
| These activities are then repeated with north pole of the magnet. What will be the observations? |
| (b) Name and define the phenomenon involved in above activities. |
| (c) Name the rule which can determine the direction of current in each case. |
| A student was given a solution to find its pH. His teacher declared his recorded pH as wrong. Student explained to his teacher, all the steps done by him while finding pH of sample. Mark the step taken by student in which he committed mistake. |
| (a) collection of apparatus. |
| (b) clearing of all apparatus |
| (c) making pH paper wet and then dip it in sample. |
| (d) recording observation. |
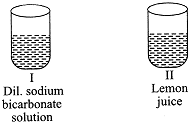
| A student was provided with four samples of solutions as shown in figures (I), (II), (III), and (IV). He determined pH value of each solution by using pH paper. The correct sequence of colour change of pH paper observed by the student will be: |
 |
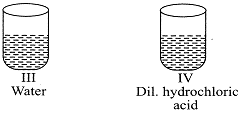 |
| (a) indigo light red green red |
| (b) red indigo green light red |
| (c) indigo red green yellow |
| (d) green red yellow indigo |
| The products of reaction between zinc and sodium hydroxide solution are: |
| (a) sodium carbonate and water |
| (b) sodium zincate and hydrogen |
| (c) zinc hydroxide and hydrogen |
| (d) zinc carbonate and hydrogen |
| A student placed Zn rod in\[\mathbf{FeS}{{\mathbf{O}}_{\mathbf{4}}}\] solution. After 10 hours when rod was taken out and it was observed that: |
| (a) Zn rod became thinner. |
| (b) Zn rod became thicker due to Iron deposition. |
| (c) Zn rod remains as it was. |
| (d) Zn rod has holes. |
| Four test tube smarked I, II, III and IV were taken 20 ml of \[\mathbf{A}{{\mathbf{l}}_{\mathbf{2}}}{{\mathbf{(S}{{\mathbf{O}}_{\mathbf{4}}}\mathbf{)}}_{\mathbf{3}}}\] solution in water was poured in each of the test tubes. A piece of zinc metal was placed in test tube I, an iron nail was put in test tube II, copper turnings were put in test tube III and a clean aluminium strip was placed was placed in test tube IV. No change was observed in any of the test tubes. The correct inference drawn is: |
| (a) Copper is more reactive than Aluminium. |
| (b) Zinc is more reactive than Aluminium. |
| (c) Zinc is more reactive than Copper. |
| (d) Zinc, Iron and Copper are less reactive than Aluminium. |
| A teacher demonstrated the experiment 'To find the equivalent resistance of two resistors when connected in series'. Rahul and Raghav after observing the experiment concluded: |
| Rahul: The current passing through the resistors in series combination is same. |
| Raghav: The potential difference across combination of resistors is the sum of potential differences across each of them. |
| Out of the options given below which one is correct? |
| (a) Rahul is right, Raghav is wrong. |
| (b) Raghav is right, Rahul is wrong. |
| (c) Both Rahul and Raghav are wrong. |
| (d) Both Rahul and Raghav are right. |
| The resistances\[{{R}_{1}}\], and\[{{R}_{2}}\], are connected in parallel. The equivalent resistance of the combination is: |
| (a) \[{{\mathbf{R}}_{\mathbf{1}}}\mathbf{+}{{\mathbf{R}}_{\mathbf{2}}}\] |
| (b) \[{{\mathbf{R}}_{\mathbf{1}}}-{{\mathbf{R}}_{\mathbf{2}}}\] |
| (c) \[\frac{{{\mathbf{R}}_{\mathbf{1}}}{{\mathbf{R}}_{\mathbf{2}}}}{{{\mathbf{R}}_{\mathbf{1}}}\mathbf{+}{{\mathbf{R}}_{\mathbf{2}}}}\] |
| (d) \[\frac{{{\mathbf{R}}_{\mathbf{1}}}\mathbf{+}{{\mathbf{R}}_{\mathbf{2}}}}{{{\mathbf{R}}_{\mathbf{1}}}{{\mathbf{R}}_{\mathbf{2}}}}\] |
| In an experiment to show that ?sunlight is necessary for photosynthesis? the leaf is boiled in alcohol for few minutes using a water bath. It is essential because: |
| (a) Alcohol is highly volatile. |
| (b) Steam from the water bath heats the leaf rapidly. |
| (c) Steam from the water dissolves the chlorophyll. |
| (d) Alcohol is flammable. |
| In the experiment to show that '\[\mathbf{C}{{\mathbf{O}}_{\mathbf{2}}}\] is released during respiration', the solution in the test tube is chemically: |
| (a) NaOH (b) KOH |
| (c) NaCl (d) KCI |
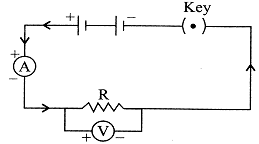
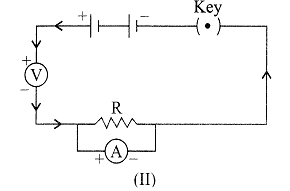
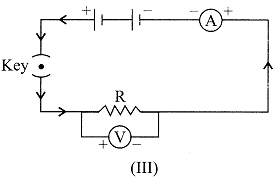
| To study the dependence of potential difference (V) on current I across Resistor (R), four circuit diagrams are prepared. |
 (I) (I) |
 |
 |
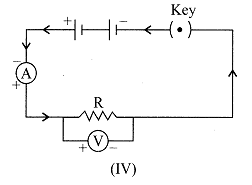 |
| (i) Select the circuit diagrams which are correct. |
| (ii) Give reason for the circuit diagrams which are not correct. |
| (i) While studying the combination reaction on adding water to quick lime, name the product formed and write its colour. |
| (ii) While studying the decompostion reaction by heating ferrous sulphate crystals in a test-tube, a product is formed in the test?tube. Name the product and write its colour. |
You need to login to perform this action.
You will be redirected in
3 sec
