| State reason for the following: |
| (i) Lemon is used for restoring the shine of tarnished copper vessels. |
| (ii) A metal sulphide is converted into its oxide to extract the metal from the sulphide ore. |
| (iii) Copper wires are used in electrical connections. |
| Select (i) combination reaction, (ii) decomposition reaction and (iii) displacement reaction from the following chemical equations: |
| (i) \[ZnC{{O}_{3}}(s)\to ZnO(s)+C{{O}_{2}}(g)\] |
| (ii) \[Pb(s)+CuC{{l}_{2}}(aq)\to PbC{{l}_{2}}(aq)+Cu(s)\] |
| (iii) \[NaBr(aq)+AgN{{O}_{3}}(aq)\to AgBr(s)+NaN{{O}_{3}}(aq)\] |
| (iv) \[{{H}_{2}}(g)+C{{l}_{2}}(g)\to 2HCl(g)\] |
| (v) \[F{{e}_{2}}{{O}_{3}}+2Al\to A{{l}_{2}}{{O}_{3}}+2Fe\] |
| (vi) \[3{{H}_{2}}(g)+{{N}_{2}}(g)\to 2N{{H}_{3}}(g)\] |
| (vii) \[CaC{{O}_{3}}(s)\xrightarrow{Heat}CaO(s)+C{{O}_{2}}(g)\] |
| State reason for the following: |
| (i) dry HCl gas does not change the colour of the dry blue litmus paper. |
| (ii) alcohol and glucose also contain hydrogen, but do not conduct electricity. |
| (iii) Cone. of \[{{H}_{3}}{{O}^{+}}\] ion is affected when a solution of an acid is diluted. |
| State the kind of chemical reactions in the following examples: |
| (i) Digestion of food in stomach |
| (ii) Combustion of coal in air |
| (iii) Heating of limestone |
| (a) State the purpose of formation of urine. |
| (b) What will happen if there is no tubular reabsorption in the nephrons of kidney. |
| Show four different ways in which four resistors of r ohm each may be connected in a circuit. In which case is the equivalent resistance of the combination. |
| (i) maximum (ii) minimum |
| Amit lives in Delhi and is much concerned about the increasing electricity bill of his house. He took some steps to save electricity and succeeded in doing so. |
| (i) Mention any two steps that Amit might have taken to save electricity. |
| (ii) Amit fulfilled his duty towards the environment by saving electricity. How? |
| (iii) Which alternative source of energy would you suggest Amit to use? |
| (a) Define corrosion. |
| (b) What is corrosion of iron called? |
| (c) How will you recognise the corrosion of silver? |
| (d) Why corrosion of iron is a serious problem? |
| (e) How can we prevent corrosion? |
| Write balanced chemical equations for the following statements: |
| (i) NaOH solution is heated with zinc granules. |
| (ii) Excess of carbon dioxide gas is passed through lime water. |
| (iii) Dilute sulphuric acid reacts with sodium carbonate. |
| (iv) Egg shells are dropped in hydrochloric acid. |
| (v) Copper (II) oxide reacts with dilute hydrochloric acid. |
| (a) Write three main functions of the nervous system. |
| (b) In the absence of muscle cells, how do plant cells show movement? |
| (a) Draw magnetic field lines of a bar magnet. "Two magnetic field lines never intersect each other." Why? |
| (b) An electric oven of 1.5 kW is operated in a domestic circuit (220 V) that has a current rating of 5 A. What result do you expect in this case? Explain. |
| What is meant by resistance of a conductor? Name and define its SI unit. List the factors on which the resistance of a conductor depends. How is the resistance of a wire affected if ? |
| (i) its length is doubled, |
| (ii) its radius is doubled? |
| (i) Establish a relationship to determine the equivalent resistance R of a combination of three resistors having resistances \[{{\mathbf{R}}_{\mathbf{1}}}\mathbf{,}\,{{\mathbf{R}}_{\mathbf{2}}}\] and \[{{\mathbf{R}}_{\mathbf{3}}}\] connected in parallel. |
| (ii) Three resistors are connected in an electrical circuit as shown. Calculate the resistance between A and B. |
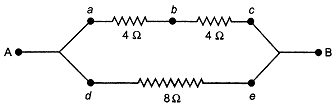 |
| Four students studied reactions of zinc and sodium carbonate with dilute hydrochloric acid and dilute sodium hydroxide solutions and presented their results as follows. The \[(\uparrow )\] shows evolution of gas and \[(-)\] shows no reaction. The right set is: | |||
| (a) | \[Zn\] | \[N{{a}_{2}}C{{O}_{3}}\] | |
| HCl | √ | √ | |
| NaOH | √ | √ | |
| (b) | \[Zn\] | \[N{{a}_{2}}C{{O}_{3}}\] | |
| HCl | - | √ | |
| NaOH | √ | √ | |
| (c) | \[Zn\] | \[N{{a}_{2}}C{{O}_{3}}\] | |
| HCl | √ | √ | |
| NaOH | √ | - | |
| (d) | \[Zn\] | \[N{{a}_{2}}C{{O}_{3}}\] | |
| HCl | √ | - | |
| NaOH | √ | √ | |
| Dilute NaOH solution and solid sodium carbonate: |
| (a) react only on heating |
| (b) react very slowly |
| (c) do not react |
| (d) react vigorously |
| The colour of Cu metal is: |
| (a) reddish brown |
| (b) blue |
| (c) green |
| (d) grey |
| Shashank was asked to carry out a displacement reaction which would show the following: |
| (i) Formation of colourless solution |
| (ii) Black deposits |
| The reactants he should use are: |
| (a) \[Fe(s)\] and \[A{{l}_{2}}{{(S{{O}_{4}})}_{3}}(aq)\] |
| (b) \[Al(s)\] and \[FeS{{O}_{4}}(aq)\] |
| (c) \[Zn(s)\] and \[CuS{{O}_{4}}(aq)\] |
| (d) \[Fe(s)\] and \[ZnS{{O}_{4}}(aq)\] |
| Mrignayani was doing the experiment of comparing reactivity of metals in the laboratory. She was given aluminium metal and was told to check reactivity by using four solutions as shown below. She would observe that reaction takes place in: |
(A) 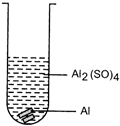 (B) (B) 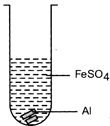 |
(C) 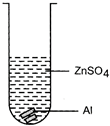 (D) (D) 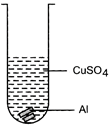 |
| (a) A and B |
| (b) B, C and D |
| (c) A, C and D |
| (d) C and D |
| In an experiment to find the equivalent resistance of a series combination of two resistance of \[3\,\Omega \] and \[4\,\Omega \] in the circuit diagram given. The circuit will give: |
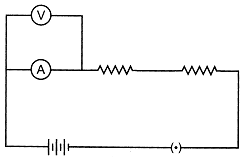 |
| (a) Incorrect reading for current I and reading for voltage V |
| (b) Incorrect readings for both current I and voltage V |
| (c) Correct reading for current I and incorrect reading for voltage V |
| (d) Correct readings for both voltage V and current |
| A Student joined three resistances as shown in the circuit below. The current recorded by ammeter (A). |
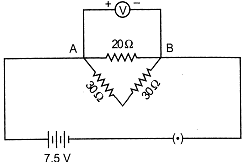 |
| (a) 0.25 A (b) 0.5 A |
| (c) 0.75 A (d) 1 A |
| The iodine solution is: |
| (a) Pure iodine dissolved in water |
| (b) Potassium iodide in water |
| (c) Iodine dissolved in potassium iodide |
| (d) Potassium iodide dissolved in iodide |
(A) 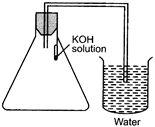 (B) (B) 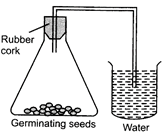 |
(C) 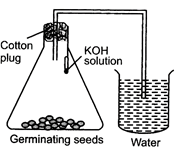 (D) (D) 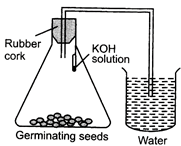 |
| Choose the correct set up to demonstrate that \[C{{O}_{2}}\] is given out during respiration: |
| (a) A |
| (b) B |
| (c) C |
| (d) D |
You need to login to perform this action.
You will be redirected in
3 sec
