A) \[x=y=z\]
B) \[x=y<z\]
C) \[x<y<z\]
D) \[x>y>z\]
Correct Answer: D
Solution :
| [d] \[Cr(24)=1{{s}^{2}},\,2{{s}^{2}},\,2{{p}^{6}},\,3{{s}^{2}},\,3{{p}^{6}},\,4{{s}^{1}},\,3{{d}^{5}}\] |
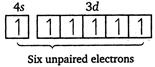 |
| \[Mn\,(25)=1{{s}^{2}},\,2{{s}^{2}},\,2{{p}^{6}},\,3{{p}^{6}},\,4{{s}^{2}},\,3{{d}^{5}}\] |
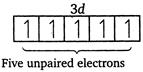 |
| \[Fe(26)=1{{s}^{2}},\,2{{s}^{2}},\,2{{p}^{6}},\,3{{s}^{2}},\,3{{p}^{6}},\,4{{s}^{2}},\,3{{d}^{6}}\] |
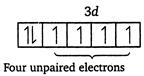 |
| Magnetic moment \[(\mu )=\sqrt{n(n+2)}\] |
| where, \[n=\] number of unpaired electrons. |
| Greater the number of unpaired electrons, greater will be the magnetic moment. |
You need to login to perform this action.
You will be redirected in
3 sec
