A) In \[Mn{{O}_{4}}^{2-}Mn\] is in intermediate oxidation state
B) In \[Mn{{O}_{4}}^{2-},Mn\] is in lowest oxidation state
C) In \[Mn{{O}_{4}}^{2-},Mn\] is in intermediate oxidation state
D) None of the above
Correct Answer: A
Solution :
| [a] In \[Mn{{O}_{4}}^{2-},\] the oxidation number of\[Mn\] is + 6. It can increase its oxidation number (to + 7) or decrease its oxidation number (to + 4, + 3, + 2, 0). Hence it undergoes disproprotion rection is acidic medium. |
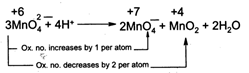 |
| In \[Mn{{O}_{4}}^{-},Mn\] is in its highest oxidation state i.e., +7. It can only decrease its oxidation number. Hence, it cannot undergo disproportionation reaction. |
You need to login to perform this action.
You will be redirected in
3 sec
