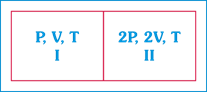
A) The Pressure in the two compartments are unequal.
B) Volume of compartment I is \[\frac{2V}{5}\]
C) Volume of compartment II is\[\frac{12\,V}{5}\]
D) Final pressure in compartment I is \[\frac{4P}{3}\]
Correct Answer: C
Solution :
| [c] In the equilibrium position the net force on the partion will be zero. |
| Hence pressure on both sides are same. Initially, PV = nRT |
| \[{{n}_{1}}=\frac{{{P}_{1}}{{V}_{1}}}{R{{T}_{1}}}=\frac{PV}{RT}\]& |
| \[\,{{n}_{2}}=\frac{\left( 2P \right)\left( 2V \right)}{RT}=4\frac{PV}{RT}\Rightarrow {{n}_{2}}=4{{n}_{1}}\] |
| Moles remains conserved. |
| Finally, pressure becomes equal in both parts. |
| Using, \[{{P}_{1}}{{V}_{1}}=\text{ }{{n}_{1}}R{{T}_{1}}\] |
| \[{{P}_{2}}{{V}_{2}}=\text{ }{{n}_{2}}R{{T}_{2}}\] |
| \[\therefore \] \[{{P}_{1}}={{P}_{2}}\And {{T}_{1}}={{T}_{2}}\] |
| \[\therefore \] \[\frac{{{V}_{1}}}{{{V}_{2}}}=\frac{{{n}_{1}}}{{{n}_{2}}}=\frac{1}{4}\,\,\,\,\Rightarrow \,\,\,{{V}_{2}}=4{{V}_{1}}\] |
| Also \[{{V}_{1}}+{{V}_{2}}=3V\] |
| \[\Rightarrow \] \[{{V}_{1}}+4{{V}_{1}}=3V\] |
| And \[{{V}_{2}}=\frac{12}{5}V\] |
| In compartment (I): |
| \[{{P}_{1}}'{{V}_{1}}=\text{ }{{n}_{1}}R{{T}_{1}}\] |
| \[{{P}_{1}}\left( \frac{3V}{5} \right)=\left( \frac{PV}{RT} \right)RT\] |
| \[{{P}_{1}}'=\frac{5PV}{3V}=\frac{5}{3}P\] |
You need to login to perform this action.
You will be redirected in
3 sec
