Answer:
When an ore on heating in air produces SO2, then the ore is a sulphide. Sulphide ores are concentrated by Froth floatation process. (1)
A concentrated sulphide ore is converted into the metal by
(i) Roasting
(ii) Reduction of oxide
Roasting The sulphide ore is heated in the presence of excess of air such that it changes to a metallic oxide.
e.g., 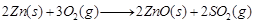 (1)
Reduction of oxide The roasted ore is reduced to metal by heating with an appropriate reducing agent such as carbon, carbon monoxide or hydrogen.
e.g.,
(1)
Reduction of oxide The roasted ore is reduced to metal by heating with an appropriate reducing agent such as carbon, carbon monoxide or hydrogen.
e.g.,  (1)
(1)
You need to login to perform this action.
You will be redirected in
3 sec
